Open Access Article
Open Access ArticleN,N-Dimethylformamide-stabilized ruthenium nanoparticle catalyst for β-alkylated dimer alcohol formation via Guerbet reaction of primary alcohols†
Tatsuki Nagataa,
Kanji Okadaa,
Ryota Kondoa,
Takashi Toyao b,
Ken-ichi Shimizu
b,
Ken-ichi Shimizu b,
Takeyuki Suzuki
b,
Takeyuki Suzuki c and
Yasushi Obora
c and
Yasushi Obora *a
*a
aDepartment of Chemistry and Materials Engineering, Faculty of Chemistry, Materials, and Bioengineering, Kansai University, Suita, Osaka 564-8680, Japan. E-mail: obora@kansai-u.ac.jp; Fax: +81-6-6339-4026; Tel: +81-6-6368-087
bInstitute for Catalysis, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan
cComprehensive Analysis Center, SANKEN, Osaka University, 8-1 Mihogaoka, Ibaraki, Osaka 567-0057, Japan
First published on 6th June 2022
Abstract
N,N-Dimethylformamide-stabilized Ru nanoparticles (NPs) provide a highly efficient catalyst for the Guerbet reaction of primary alcohols. DMF-modified Ru NPs were synthesized, and characterized by transition electron microscopy, and X-ray absorption spectroscopy, X-ray photoelectronspectroscopy, and Fourier-transform infrared spectroscopy. The Ru NP catalyst was highly durable during catalytic reactions under external additive/solvent-free conditions.
Introduction
Catalytic hydrogen borrowing has attracted considerable interest as a green and atom-economical alkylation process.1 Owing to the importance of sustainability, minimizing the production of undesired byproducts is important.2 The Guerbet reaction, which was developed in the 1890s by Marcel Guerbet,3 produces Guerbet alcohols via dehydrative condensation of aliphatic alcohols. Such alcohols are widely used in surfactants, lubricants, and personal care products.4Heterogeneous catalysts,5 e.g., metals,6 metal oxides,7 and metal–organic frameworks,8 for this transformation have been developed. The harsh reaction conditions required for this reaction and its low selectivity have motivated researchers to develop more-efficient methods for the Guerbet reaction. The use of homogeneous catalysts, e.g., Ir-,9 Ru-,10 and Mn11-based catalysts, has been investigated. However, the cost and ease of synthesis and handling of highly active ligands and additives have been overlooked.
An alternative approach involves the development of colloidal transition-metal nanoparticle (M NP) catalysts that can be readily prepared via a one-step process.12 Our group has focused on developing a simple method for the synthesis of N,N-dimethylformamide (DMF)-stabilized M NPs and their use in catalytic reactions. In this method, DMF is used as a reductant, protectant, and solvent. Among various M NPs,13 Ir NPs show high catalytic activities in the β-benzylation of linear alcohols and β-dimethylation of secondary alcohols with methanol via a hydrogen-borrowing process.14 However, the low abundance and high cost of Ir are drawbacks of this method.
Here, we report the synthesis of DMF-stabilized Ru NPs, their structural characterization, and their use as a catalyst in the Guerbet reaction (homo-β-alkylation) of primary alcohols.
Results and discussion
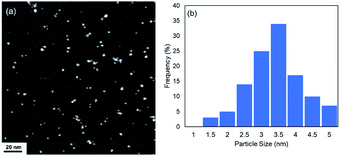
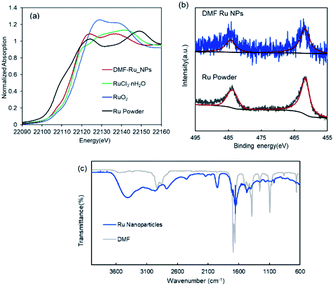
The DMF-stabilized Ru NPs were synthesized as follows. RuCl3·nH2O was dissolved in hydrochloric acid solution. DMF (50 mL) was added to a 300 mL three-necked round-bottomed flask, and the solution was preheated to 140 °C (±2 °C) and stirred for 5 min. Then a 0.1 M RuCl3·nH2O hydrochloric solution (RuIII) was added to the hot DMF solution. The mixture was stirred (1500 rpm) at 140 °C (±2 °C) for 10 h. The brown solution afforded Ru NPs. The annular dark-field scanning transmission electron microscopy (ADF-STEM) image in Fig. 1 shows the formation of Ru NPs of mean diameter 3.2 nm.The electronic state of the Ru species in the DMF-stabilized Ru NPs was clarified. The X-ray absorption near-edge structure (XANES) spectrum was compared with those of metallic Ru powder and RuO2 (Fig. 2a). The absorption edge position of the DMF-stabilized Ru NPs lay between those for metallic Ru and RuO2, which indicates that reduced Ru species were formed by reduction of the precursor (RuIII). Fig. 2b shows the X-ray photoelectron spectroscopy (XPS) results for the DMF-stabilized Ru NPs. Ru signals ascribed to Ru 3p3/2, 3p1/2,and 3d5/2 are present at 461.5, 444.2, and 280.8 eV, respectively; in the Ru powder spectrum, the signals appear at 461.2 and 279.9 eV, respectively in addition C 1s signals were detected at 284.2, 284.9, 286.0 (Fig. 2b and S1†).15 Formation of the DMF-stabilized Ru NPs by reduction of the Ru precursor is consistent with the XANES analysis. Fourier-transform infrared (FT-IR) spectra were recorded. Fig. 2c shows the Ru NP spectrum after DMF removal. The absorption peaks for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1652 cm−1) and C–N (1390 cm−1) vibrational modes of DMF in the spectrum of the DMF-stabilized Ru NPs differ from those in the spectrum of the DMF solvent. The IR absorption attributed to metal carbonyl in the absence of DMF appears at approximately 1950 cm−1. This suggests that CO generated from DMF interacts with metal species.16
O (1652 cm−1) and C–N (1390 cm−1) vibrational modes of DMF in the spectrum of the DMF-stabilized Ru NPs differ from those in the spectrum of the DMF solvent. The IR absorption attributed to metal carbonyl in the absence of DMF appears at approximately 1950 cm−1. This suggests that CO generated from DMF interacts with metal species.16
In this process, DMF acts as a reducing agent at above 100 °C in metal nanoparticle synthesis and the DMF molecules strongly coordinate to the metal center and stabilize the Ru NPs.13
Thermogravimetric (TG) analysis was used to investigate the thermal stability of the Ru NPs (Fig. S2†). The TG curves in the range 50–400 °C suggest that the DMF-stabilized Ru NPs were stabilized by various molecules on the surface. The 5% weight loss temperature of the Ru NPs was 190 °C, whereas the decomposition temperature of Ru3(CO)12 was reported in the 160–220 °C range.17 The decomposition products were identified by thermogravimetry-electron ionization high-resolution time-of-flight mass (TG-EI-HR-TOF MS) analysis of the Ru NPs; H2O, DMF, CO, CO2, and dimethylamine were detected (Fig. S3–S5†). These compounds were derived from the Ru NPs. DMF decomposes to give dimethylamine, CO2 and CO.18 Four ions (DMF, dimethylamine, CO2, and CO) were used to analyze the thermal behavior of the Ru NPs. The extracted ion chromatograms of these ions are shown in Fig. S5.† Dimethylamine, which was released around 100–550 °C, is a product of DMF thermal decomposition. DMF was also released at 100–280 °C. Dimethylamine and DMF served as a part of stabilizer. On heating the Ru NPs, a portion of the stabilizers DMF and dimethylamine were expected to be liberated from the Ru metal, generating Ru NPs with partially open sites that could act as active catalysts. Considerable attention must be given to the temperature at which CO is detected because it can also be formed via CO2 fragmentation. CO was detected, not simultaneously with CO2 and DMF, at approximately 280 °C and above 400 °C. These results suggest partial CO desorption from the Ru NPs.
Next, we investigated the Guerbet reaction of 1-dodecanol (1a) as a model substrate under various conditions (Table 1). The reaction of 1a (2 mmol) was performed in the presence of Ru NPs (0.05 mol%), with KOtBu (0.2 mmol) as a base, at 150 °C for 24 h; the main β-alkylated product 2a was obtained in a moderate yield of 93% and with 98% selectivity (Table 1, entry 1). The effect of the base was then determined. A strong base such as KOH gave the desired 2a in 83% yield, whereas weak inorganic bases such as Cs2CO3 and K2CO3 were ineffective (Table 1, entries 2–4). Other Ru complexes gave the desired product in moderate yields (Table 1, entries 5–7). Use of the M NP precursor (RuCl3·H2O) as the catalyst resulted in a low yield and selectivity (Table 1, entry 8). At this time, the metal black formation significantly decreased catalytic activity. The reaction did not proceed in the absence of a base (Table 1, entry 9) and Ru NPs (Table 1, entry 10). This shows that the catalyst and base are both necessary for alkylation.
| Entry | Ru catalyst | Base | Conv. (%) | Yieldb (%) (selectivity, %) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2 mmol) was reacted in the presence of a Ru catalyst (0.05 mol%) and base (0.2 mmol) at 150 °C for 24 h.b GC yield based on 1a. Numbers in square brackets show isolated yields. n.d. = not detected by GC. Numbers in parentheses show selectivity of 2a = [yield (%) 1a]/[conv. (%) 1a]. | ||||
| 1 | Ru NPs | KOtBu | 95 | 93 (98) [83] |
| 2 | Ru NPs | KOH | 85 | 83 (98) |
| 3 | Ru NPs | Cs2CO3 | 12 | 2 (17) |
| 4 | Ru NPs | K2CO3 | 15 | 3 (20) |
| 5 | [Ru(p-cymene)Cl2]2 | KOtBu | 35 | 30 (86) |
| 6 | RuCl2(PPh3)3 | KOtBu | 60 | 55 (92) |
| 7 | Ru3CO12 | KOtBu | 47 | 28 (60) |
| 8 | RuCl3·nH2O | KOtBu | 40 | 33 (83) |
| 9 | Ru NPs | None | <1 | n.d. |
| 10 | None | KOtBu | 5 | Trace |
The reaction scope was investigated under the optimal reaction conditions (Table 1, entry 1); the results are shown in Fig. 3. The reaction with any C16 to C4 aliphatic primary alcohols gave the corresponding Guerbet alcohols (2b–2i). Aliphatic alcohols with short carbon chains resulted in low yields. Similarly, 1-cyclohexylpropanol and tetrahydrogeraniol were efficiently alkylated to give 2j and 2k in good yields (74% and 64%, respectively).
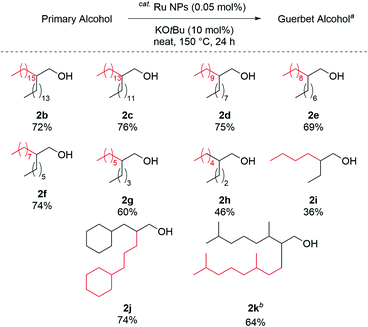 | ||
| Fig. 3 Substrate scope. a Reaction conditions, Table 1, entry 1. b 72 h. | ||
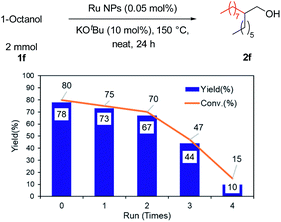
The reusability of the Ru NPs was investigated (Fig. 4). The substrates and desired products were removed by distillation (40 Pa, 130 °C). The base was then removed from the mixture by filtering through a cotton plug (for details see Fig. S6†). The Ru NPs retained their catalytic activity and selectivity over several cycles without the need for preactivation.
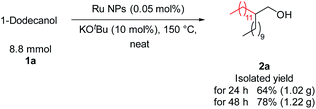
The feasibility of this transformation was demonstrated by the gram-scale synthesis of 2a (Fig. 5). Specifically, 1a (8.8 mmol) was stirred at 150 °C for 48 h in the presence of Ru NPs (0.05 mol%), KOtBu (0.88 mmol, 10 mol%), which afforded 2a in good yield (1.22 g). This result supports the scalability of the process.
The Ru NPs were characterized by STEM, X-ray absorption spectroscopy, and XPS to elucidate the reasons for their high catalytic activity. After the 1st reaction, the Ru NP mean diameter had increased slightly to 4.4 nm (Fig. 6a and S7†). The XANES spectrum of the used Ru NPs was similar to that of the fresh Ru NPs (Fig. 6b, green). The Ru 3p3/2, Ru 3p1/2, 3d5/2 peaks of the used Ru NPs appeared at 461.4, 483.7, 280.1 eV, respectively (Fig. S8†). These results suggest that the Ru species in the DMF-stabilized Ru NPs were retained after several uses. The DLS analysis showed that the size of Ru NPs increased after 4th reuse (Fig. S9†). The size increase and aggregation of the metal nanoparticles would decrease the catalytic activity during the recycling process.19,20
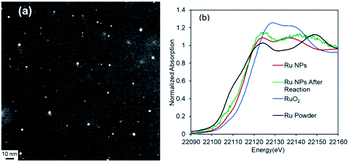 | ||
| Fig. 6 (a) ADF-STEM image of Ru NPs after reaction and (b) Ru K-edge XANES spectra of Ru NPs before (red) and after reaction (green). | ||
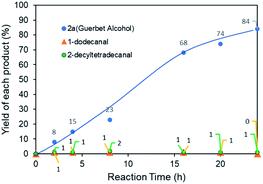
Fig. 7 shows the time-dependent production of intermediates and the desired products during the Guerbet reaction. The yield of 2a gradually increased, with low yields of reaction intermediates such as 1-dodecanal and 1-decyltetradecanal. The produced aldehyde intermediates were immediately consumed. Use of the Ru NP catalyst minimized byproduct formation because of their high dehydrogenation/hydrogenation properties in the Guerbet reaction.7a,8b
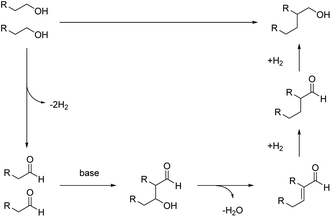
Based on our experiments, a plausible catalytic mechanism is proposed for the β-alkylation of primary alcohols. As shown in Fig. 8, the primary alcohol is oxidized to give an aldehyde. In the presence of a base, the initially formed aldehydes condense to give the unsaturated aldehyde (aldol condensation). The α,β-unsaturated aldehyde then undergoes hydrogenation in the presence of Ru NPs to generate the desired Guerbet alcohol.
Conclusions
β-Alkylation of primary alcohols to branched alcohols under mild reaction conditions was achieved by using a DMF-stabilized Ru NP catalyst. The Ru NP catalyst promoted the Guerbet reaction under external ligand- and solvent-free conditions. Various primary alcohols were converted into the corresponding branched alcohols in high yields. This catalytic system has significant advantages, namely mild operating conditions, a simple catalyst preparation procedure, high catalyst reusability, and a broad substrate scope. Its use will enable green sustainable Guerbet reactions to be performed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was performed by JSPS KAKENHI Grant number 19K05573 and the Research Program for Next Generation Young Scientists of “Five-star Alliance” in “NJRC Mater. & Dev.”. High-resolution mass spectra were measured Global Facility Center, Hokkaido University. We thank Y. Murakami and T. Ishibashi of the members of the Comprehensive Analysis Center, and N. Eguchi of the Center of Scientific Instrument Renovation and Manufacturing Support, SANKEN, Osaka University, for TEM, ICP-AES analyses and JEOL Ltd for TG TOF-MS analyses. X-ray absorption measurements were performed at the BL14B2 beamline of SPring-8 at the Japan Synchrotron Radiation Research Institute (JASRI) (No. 2019A1614).Notes and references
- (a) Y. Obora, ACS Catal., 2014, 4, 3972 CrossRef CAS; (b) B. G. Reed-Berendt, D. E. Latham, M. B. Dambatta and L. C. Morrill, ACS Cent. Sci., 2021, 7, 570 CrossRef CAS PubMed; (c) K.-I. Shimizu, Catal. Sci. Technol., 2015, 5, 1412 RSC; (d) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410 CrossRef CAS PubMed.
- (a) H. Li, A. Riisager, S. Saravanamurugan, A. Pandey, R. S. Sangwan, S. Yang and R. Luque, ACS Catal., 2018, 8, 148 CrossRef CAS; (b) A. Kumar, P. Daw and D. Milstein, Chem. Rev., 2022, 122, 385 CrossRef CAS PubMed; (c) S. Shylesh, A. A. Gokhale, C. R. Ho and A. T. Bell, Acc. Chem. Res., 2017, 50, 2589 CrossRef CAS PubMed; (d) L. Wu, T. Moteki, A. A. Gokhale, D. W. Flaherty and F. D. Toste, Chem, 2016, 1, 32 CrossRef CAS.
- (a) M. Guerbet, C. R. Hebd. Seances Acad. Sci., 1899, 128, 511 Search PubMed; (b) A. J. O'Lenick, J. Surfactants Deterg., 2001, 4, 311 CrossRef.
- L. D. Rhein, M. Schlossman, A. J. O'Lenick and P. Somasundaran, Surfactants in Personal Care Products and Decorative Cosmetics, Taylor & Francis Group, 2007 Search PubMed.
- (a) D. Gabriëls, W. Y. Hernández, B. Sels, P. Van Der Voort and A. Verberckmoes, Catal. Sci. Technol., 2015, 5, 3876 RSC; (b) J. T. Kozlowski and R. J. Davis, ACS Catal., 2013, 3, 1588 CrossRef CAS.
- (a) M. Utsunomiya, R. Kondo, T. Oshima, M. Safumi, T. Suzuki and Y. Obora, Chem. Commun., 2021, 57, 5139 RSC; (b) X. Zhang, Z. Liu, X. Xu, H. Yue, G. Tian and S. Feng, ACS Sustainable Chem. Eng., 2013, 1, 1493 CrossRef CAS; (c) K. A. Goulas, S. Sreekumar, Y. Song, P. Kharidehal, G. Gunbas, P. J. Dietrich, G. R. Johnson, Y. C. Wang, A. M. Grippo, L. C. Grabow, A. A. Gokhale and F. D. Toste, J. Am. Chem. Soc., 2016, 138, 6805 CrossRef CAS PubMed.
- (a) V. N. Panchenko, E. A. Paukshtis, D. Y. Murzin and I. L. Simakova, Ind. Eng. Chem. Res., 2017, 56, 13310 CrossRef CAS; (b) S. Hanspal, Z. D. Young, H. Shou and R. J. Davis, ACS Catal., 2015, 5, 1737 CrossRef CAS; (c) O. V. Larina, K. V. Valihura, P. I. Kyriienko, N. V. Vlasenko, D. Y. Balakin, I. Khalakhan, T. Čendak, S. O. Soloviev and S. M. Orlyk, Appl. Catal., A, 2019, 588, 117265 CrossRef.
- (a) C. N. Neumann, M. T. Payne, S. J. Rozeveld, Z. Wu, G. Zhang, R. J. Comito, J. T. Miller and M. Dincă, ACS Appl. Mater. Interfaces, 2021, 13, 52113 CrossRef CAS PubMed; (b) C. N. Neumann, S. J. Rozeveld and M. Dincă, ACS Catal., 2021, 11, 8521 CrossRef CAS.
- (a) T. Matsu-ura, S. Sakaguchi, Y. Obora and Y. Ishii, J. Org. Chem., 2006, 71, 8306 CrossRef CAS PubMed; (b) K. Koda, T. Matsu-ura, Y. Obora and Y. Ishii, Chem. Lett., 2009, 38, 838 CrossRef CAS; (c) G. Xu, T. Lammens, Q. Liu, X. Wang, L. Dong, A. Caiazzo, N. Ashraf, J. Guan and X. Mu, Green Chem., 2014, 16, 3971 RSC; (d) S. Chakraborty, P. E. Piszel, C. E. Hayes, R. T. Baker and W. D. Jones, J. Am. Chem. Soc., 2015, 137, 14264 CrossRef CAS PubMed.
- (a) G. R. M. Dowson, M. F. Haddow, J. Lee, R. L. Wingad and D. F. Wass, Angew. Chem., Int. Ed., 2013, 52, 9005 CrossRef CAS PubMed; (b) T. A. DiBenedetto and W. D. Jones, Organometallics, 2021, 40, 1884 CrossRef CAS; (c) R. L. Wingad, P. J. Gates, S. T. G. Street and D. F. Wass, ACS Catal., 2015, 5, 5822 CrossRef CAS.
- (a) N. V. Kulkarni, W. W. Brennessel and W. D. Jones, ACS Catal., 2018, 8, 997 CrossRef CAS; (b) A. M. King, H. A. Sparkes, R. L. Wingad and D. F. Wass, Organometallics, 2020, 39, 3873 CrossRef CAS PubMed; (c) Y. Liu, Z. Shao, Y. Wang, L. Xu, Z. Yu and Q. Liu, ChemSusChem, 2019, 12, 3069 CrossRef CAS PubMed.
- (a) Z. B. Shifrina, V. G. Matveeva and L. M. Bronstein, Chem. Rev., 2020, 120, 1350 CrossRef CAS PubMed; (b) L. Lu, S. Zou and B. Fang, ACS Catal., 2021, 11, 6020 CrossRef CAS; (c) H. Kawasaki, Nanotechnol. Rev., 2013, 2, 5 CAS.
- T. Nagata and Y. Obora, ACS Omega, 2020, 5, 98 CrossRef CAS PubMed.
- (a) K. Oikawa, S. Itoh, H. Yano, H. Kawasaki and Y. Obora, Chem. Commun., 2017, 53, 1080 RSC; (b) M. Kobayashi, H. Yamaguchi, T. Suzuki and Y. Obora, Org. Biomol. Chem., 2021, 19, 1950 RSC.
- D. J. Morgan, Surf. Interface Anal., 2015, 47, 1072–1079 CrossRef CAS.
- (a) X. Wang, Y. Hong, H. Shi and J. Szanyi, J. Catal., 2016, 343, 185 CrossRef CAS; (b) S. Y. Chin, C. T. Williams and M. D. Amiridis, J. Phys. Chem. B, 2006, 110, 871 CrossRef CAS PubMed.
- Y. Zhang, C. Zuo, C. Li, X. Guo and S. Zhang, Green Chem., 2016, 18, 4704 RSC.
- (a) T. S. Rodrigues, M. Zhao, T.-H. Yang, K. D. Gilroy, A. G. M. da Silva, P. H. C. Camargo and Y. Xia, Chem.–Eur. J., 2018, 24, 16944 CrossRef CAS PubMed; (b) I. Pastoriza-Santos and L. M. Liz-Marzán, Langmuir, 1999, 15, 948 CrossRef CAS; (c) J. Y. Yu, S. Schreiner and L. Vaska, Inorg. Chim. Acta, 1990, 170, 145 CrossRef CAS.
- T. W. Hansen, A. T. Delariva, S. R. Challa and A. K. Datye, Acc. Chem. Res., 2013, 46, 1720 CrossRef CAS PubMed.
- K. Tabaru, M. Nakatsuji, S. Itoh, T. Suzuki and Y. Obora, Org. Biomol. Chem., 2021, 19, 3384 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compound characterization data. See https://doi.org/10.1039/d2ra02381d |
| This journal is © The Royal Society of Chemistry 2022 |