How did the bacterial community respond to the level of urbanization along the Yangtze River?†
Yi
Li
,
Luhuan
Fan
,
Wenlong
Zhang
 *,
Xiaoxiao
Zhu
,
Mengting
Lei
and
Lihua
Niu
*,
Xiaoxiao
Zhu
,
Mengting
Lei
and
Lihua
Niu
Key Laboratory of Integrated Regulation and Resource Development on Shallow Lake of Ministry of Education, College of Environment, Hohai University, Nanjing 210098, China. E-mail: zhangwenlong@hhu.edu.cn; Fax: +86-25-83786251; Tel: +86-25-83786251
First published on 20th November 2019
Abstract
Bacterial communities in the sediment of the Yangtze River influenced by rapid urbanization have thus far been under-investigated despite the importance of microorganisms as mass transporters. Here, the response patterns of the bacterial community along the Yangtze River to different levels of urbanization were generated using 16S rRNA Miseq sequencing. The results reveal that economic aspects have made the largest contribution (41.8%) to the urbanization along the Yangtze River. A clear declining tendency in the abundance of Chloroflexi and Acidobacteria and a significant increase in the abundance of Bacteroidetes were observed with an elevated urbanization level gradient. Bacterial diversity showed a negative relevance (P < 0.01) to the demographic, economic and social urbanization index. Per capita gross domestic product (GDP) (PCGDP) and the GDP of tertiary industry (GDP3) exhibited significantly (P < 0.05) negative correlations with the bacterial diversity, while a positive relationship between the pH and α-diversity (P < 0.05) was observed. Redundancy analysis revealed that PCGDP was significantly correlated (13.9%, P < 0.01) with the overall bacterial compositions, followed by temperature (10.8%, P < 0.01) and GDP3 (8.4%, P < 0.05). Meanwhile, the GDP3 (35.9%), the ratio of total nitrogen and total phosphorus (N/P) (12.9%) and the PCGDP (8.8%) were revealed to be most significantly related to the metabolic bacteria (P < 0.05). The metabolic functions of the bacteria related to the N-cycle and S-cycle were significant in the sediment of the Yangtze River. The variations of the bacterial community and metabolic function responding to the rapid urbanization were related to the economic development via the influence of the ‘mass effect’. In brief, the tertiary industry was significantly correlated with the variations in the composition of the metabolic community and the variations in the overall bacteria were both related to the tertiary and secondary industry.
Environmental significanceThe Yangtze River is the third longest river in the world and provides large numbers of freshwater resources. Safeguarding the water security and ecological function of the Yangtze River is important. Understanding the spatiotemporal patterns of bacterial communities along the Yangtze River benefits this, as the bacterial communities play an essential role in the transformation of environmental substances, especially nitrogen transformation and sulfur transformation. Considering that the rapid economic development and exploding population related to urban agglomeration have caused excessive consumption of resources and the discharge of a great deal of pollutants, the distribution of bacterial communities in response to the urbanization level along the Yangtze River was investigated in this study. The results show that the tertiary industry was significantly correlated with variation in the composition of the metabolic community and that variations in the overall bacteria were related to both the tertiary and secondary industry. This study could provide theoretical support for the management of sustainable development of the Yangtze River watershed. |
1. Introduction
Rivers, as the transformers of natural substances and anthropogenic pollutant loads, play significant roles in connecting the continental shelf, watersheds and the seas. Recently, substantial expansion of extraneous matters derived from the activities of people has posed strong effects on the biogeochemical cycles of the aquatic ecosystem.1 Being an important component of the aquatic ecosystem, the microbial community is very sensitive to environmental changes and governs key processes such as degradation,2 mineralization and decomposition3–5 in aquatic ecosystems and biogeochemical cycles.6,7 River sediments, with higher biological activity than the aquatic environment,8 have the most diverse microbial habitats9 especially for bacteria. Sediment bacteria are reported to play unquestionably effective roles in transforming elemental nutrients and contributing to the energy flow.10,11 Bacterial assemblages are sensitive to anthropogenic disturbances, which attracted our attention to investigate the function of bacteria in the overall soil conditions, as well as the water quality .12,13 Hence, understanding the response of bacterial diversity and community composition to anthropogenic disturbances, as well as the potential connection between the bacterial community and heterogeneous habitat in the aquatic environment is of great significance.4Spatiotemporal patterns in the distribution of microorganisms are closely related to their habitat characteristics. It is noticeable that the environmental disturbance is definitely correlated to human activities. In most rivers, overloaded exterior substances caused by direct and indirect inputs1 change the water quality and make the habitat heterogeneous for sediment microbes. Moreover, spatiotemporal variations in sediment microbes are impressionable to anthropogenic disturbance.13 As a cause of vast anthropogenic disturbances, urbanization substantially alters the physicochemical properties of aquatic environments and results in the loss of microbial biodiversity and ecological function.14,15 However, the influence factors of urbanization are concentrated not only on the human population,1 but also on the land use, economic development levels and so on. Previous research on the effects of the urbanization level considering different aspects on the sediment bacteria of the Yangtze River are still insufficient. Therefore, fully understanding the microbial community variations based on the influence of urbanization by considering different aspects of urban development would give us new insights into the anthropogenic effects on the aquatic ecosystem.
The Yangtze River is the third longest river in the world, and provides large numbers of freshwater resources. Recently, a remarkable phenomenon in urban agglomeration along the Yangtze River has been confirmed to influence the stability of the river ecosystems.16 The rapid economic development and exploding population related to the urban agglomeration have caused excessive consumption of resources and the discharge of a great deal of pollutants,17 leading to significant pressure on the environment and eventually a decrease in the biodiversity in the aquatic ecosystems.18 With the exception of studies on metal pollution19–21 or excess nutrient loads22,23 of the Yangtze River, most researches mainly focused on the nitrogen cycling microbes or sediment bacteria at the Yangtze River estuary.24,25 However, to the best of our knowledge, no comprehensive study has paid attention to the microbial communities in the Yangtze River sediment under the influence of rapid urbanization based on different urbanization aspects.
Culture independent gene sequencing provides a new insight into microbial diversity.26–28 In this study, a high-throughput sequencing technology was applied to explore the phylogenetic diversity patterns of microbes in the sediment of the Yangtze River. The objectives of the study were to: (1) explore the bacterial diversity and community compositions in the sediment along the Yangtze River; (2) evaluate the urbanization level along the Yangtze River and illuminate the potential relationships between the spatiotemporal variation of bacteria communities and the urbanization levels; and (3) provide theoretical support to repair the water quality of the Yangtze River and manage the sustainable development of the Yangtze watershed.
2. Materials and methods
2.1 Study site and sample collection
The Yangtze River is situated in China and originates from the plateau area, which is 6300 km long and is a significant resource of industrial, agricultural and domestic water supplies for the Yangtze basin. The level of urbanization is on the rise along the Yangtze River from upstream to downstream. According to Changjiang & Southwest Rivers water resources bulletin, 925 billion cubic meters of water flowed into the sea from the Yangtze River basin in 2014. The total water supply is 12.27 billion cubic meters, of which 14% is domestic water, 35.2% is industrial water, 49.8% is agricultural water, and 1% is ecological water. The total water consumption is 84.58 billion cubic meters, with a comprehensive water consumption rate of 42%. Sewage discharge is 33.88 billion tons. Along the river, a variety of state-controlled sections were set up as standard sampling sites to monitor hydrological condition and water quality. Samples were collected in December 2014 and July 2015 from 12 controlled sections undergoing rapid urban agglomeration along the Yangtze River (Fig. 1). The water samples were collected in five replicates at each cross-section along the Yangtze River using a plexiglass water sampler (PAS-1A, Changzhou pun sen electronic instrument factory, Changzhou, China) which was washed with local river water before each sampling to avoid potential contamination. Water samples were firstly homogenized. Then the water samples for physicochemical analysis were stored in a sterile sampling bottle at −4 °C, transported to the laboratory and physicochemical analysis was performed within 24 h. The water samples for high-throughput sequencing (1 liter) were filtered with a 0.45 μm membrane using a sterile suction flask immediately and the filter membranes were stored at −80 °C until DNA extraction. The sediment samples were collected from the surface of the river bed using a grab sampler (XDB0201, Changzhou pun sen electronic instrument factory, Changzhou, China). The sediment attached to the grab sampler was separated using sterile tools to avoid potential contamination. Each sample was split into two parts, with one stored at −4 °C for analysis of the physicochemical properties and the other stored at −80 °C in the lab for bacterial community characterization. At each section, five signal samples were collected randomly over a 20 m length strip of the surface sediment and finally a composite was obtained as the target sediment sample. Samples were initially passed through a 2 mm sieve to remove the visible plant particles and then transported to the lab as soon as possible using a container with nitrogen to isolate the samples from air. Each sample was split into two parts, with one stored at −4 °C for physicochemical properties analysis and the other stored at −80 °C in the lab for bacterial molecular genomic determination.2.2 Establishment of the index system of urbanization
All required data were taken from the Statistical Year-book (2015) in the cities of Yibin, Chongqing, Xiantao, Wuhan, Huangshi, Maanshan, Nanjing, Zhenjiang, Nantong, Suzhou and Shanghai, respectively. To accurately estimate the urban development intensity of the 12 cities, a preliminary urbanization index system was established based on a comprehensive index system introduced in a previous study using the variables listed in the previously conducted investigations29,30. The index system of urbanization established in the study contains four first-grade indicators (i.e., demographic, spatial, economic and social aspects) and eight basic indicators, which were selected by comparing their correlation coefficients and significance levels. The data pre-processing was carried out by standardizing the data using eqn (1) and (2) to eliminate the influence of the dimensions, magnitude, and positive and negative orientation.29Positive indicator:
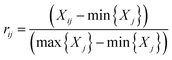 | (1) |
Negative indicator:
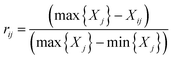 | (2) |
In which Xij denotes the value of the indicator j in year i, and max {Xj} and min {Xj} are the maximum value and minimum value of the j indicator in all years, respectively. Thus, all of the index values will fall within the range [0,1]. The weight of each index was calculated according to the information entropy and to variations in the indicators. The steps for the calculation are shown as follows:
The proportion of the indicator j in year i:
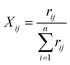 | (3) |
Information entropy of the indicator:
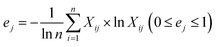 | (4) |
Entropy redundancy:
| fj = 1 − ej | (5) |
Weight of the indicator:
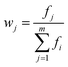 | (6) |
Evaluation of a single indicator:
| Yij = wj ×=rij | (7) |
Comprehensive level in year i:
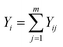 | (8) |
All indices were further selected through principal component analysis and the correlation coefficients and significance level analysis using SPSS 20.0. Table 1 summarizes the four first grade indices and eight basic grade indices selected as the final urbanization index level values system. The level of urbanization was analyzed using the entropy method as introduced in the previous study29 and the results are showed in Table 2.
| First grade index | Weight (%) | Basic grade index | Weight (%) |
|---|---|---|---|
| Demographic aspect | 12.4 | Percentage of nonagricultural population (%) | 6.7 |
| Urban population density (persons per km2) | 5.7 | ||
| Spatial aspect | 35.7 | Number of built-up areas (km2) | 14.6 |
| Number of built-up areas per capita (m2 per person) | 21.1 | ||
| Economic aspect | 41.8 | Per capita GDP (104 yuan per person) | 12.2 |
| GDP3 (108 yuan) | 19.8 | ||
| Total fixed asset investment per capita (104 yuan per person) | 9.8 | ||
| Social aspect | 10.1 | Number of phones per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people 000 people |
10.1 |
| YB | CT | WX | XT | HK | HS | MA | NJ | ZJ | NT | XL | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a YB: Yibin; CT: Cuntan; WX: Wanxian; XT: Xiantao; HK: Hankou; HS: Huangshi; MA: Maanshan; NJ: Nanjing; ZJ: Zhenjiang; NT: Nantong; XL: Xuliujing; SD: Shidongkou. b Different urbanization rank represents different values. Low: 0.065–0.202; lower middle: 0.202–0.338; upper middle: 0.338–0.475; high: 0.475–0.611. The urbanization rank was distinguished by the overall urbanization level values. | |||||||||||||
| Overall | Urbanization level values | 0.316 | 0.377 | 0.110 | 0.065 | 0.426 | 0.198 | 0.190 | 0.438 | 0.300 | 0.391 | 0.532 | 0.611 |
| First grade | Demographic aspect | 0.000 | 0.056 | 0.062 | 0.048 | 0.102 | 0.051 | 0.047 | 0.081 | 0.042 | 0.045 | 0.105 | 0.114 |
| Spatial aspect | 0.306 | 0.172 | 0.014 | 0.011 | 0.041 | 0.085 | 0.018 | 0.064 | 0.033 | 0.192 | 0.032 | 0.069 | |
| Economic aspect | 0.002 | 0.132 | 0.018 | 0.007 | 0.206 | 0.042 | 0.108 | 0.231 | 0.186 | 0.114 | 0.295 | 0.327 | |
| Social aspect | 0.008 | 0.018 | 0.015 | 0.000 | 0.077 | 0.019 | 0.017 | 0.062 | 0.039 | 0.040 | 0.100 | 0.101 | |
| Basic grade | Percentage of nonagricultural population (%) | 0.000 | 0.020 | 0.024 | 0.007 | 0.046 | 0.035 | 0.015 | 0.055 | 0.020 | 0.038 | 0.051 | 0.067 |
| Urban population density (person per km2) | 0.000 | 0.036 | 0.039 | 0.041 | 0.057 | 0.016 | 0.032 | 0.026 | 0.022 | 0.006 | 0.054 | 0.047 | |
| Number of built-up areas (km2) | 0.095 | 0.146 | 0.001 | 0.000 | 0.037 | 0.022 | 0.003 | 0.045 | 0.006 | 0.101 | 0.028 | 0.069 | |
| Number of built-up areas per capita (m2 per person) | 0.211 | 0.025 | 0.013 | 0.011 | 0.004 | 0.063 | 0.015 | 0.019 | 0.027 | 0.091 | 0.004 | 0.000 | |
| Per capita GDP (104 yuan per person) | 0.000 | 0.013 | 0.009 | 0.006 | 0.065 | 0.012 | 0.022 | 0.083 | 0.069 | 0.035 | 0.122 | 0.100 | |
| GDP3 (108 yuan) | 0.002 | 0.087 | 0.002 | 0.000 | 0.055 | 0.002 | 0.004 | 0.063 | 0.018 | 0.032 | 0.077 | 0.198 | |
| Total fixed asset investment per capita (104 yuan per person) | 0.000 | 0.032 | 0.008 | 0.001 | 0.086 | 0.028 | 0.082 | 0.085 | 0.098 | 0.047 | 0.096 | 0.030 | |
Number of phones per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people 000 people |
0.008 | 0.018 | 0.015 | 0.000 | 0.077 | 0.019 | 0.017 | 0.062 | 0.039 | 0.040 | 0.100 | 0.101 | |
| Overall | Urbanization rankb | Lower middle | Upper middle | Low | Low | Upper middle | Low | Low | Upper middle | Lower middle | Upper middle | High | High |
2.3 Environmental physicochemical properties analysis
Water temperature and soil pH (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) soil ratio of 2.5
soil ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were determined in situ using a multiparameter water quality analyzer (HQ30d, Hach, USA) and a digital pH meter, respectively. The total nitrogen (TN) was determined by direct combustion with an automated dry-combustion analyzer (LECO CNS 2000, MI, USA). Total phosphorus (TP) was measured by spectrophotometry after digestion. The sediment organic matter3 content was analyzed using the method provided in a previous study.31 Copper (Cu), cadmium (Cd), lead (Pb) and arsenic (As) were determined using the spectrophotometric method, and mercury (Hg) was analyzed using a Mercury Analyzer (MA-800, Taiwan).
1) were determined in situ using a multiparameter water quality analyzer (HQ30d, Hach, USA) and a digital pH meter, respectively. The total nitrogen (TN) was determined by direct combustion with an automated dry-combustion analyzer (LECO CNS 2000, MI, USA). Total phosphorus (TP) was measured by spectrophotometry after digestion. The sediment organic matter3 content was analyzed using the method provided in a previous study.31 Copper (Cu), cadmium (Cd), lead (Pb) and arsenic (As) were determined using the spectrophotometric method, and mercury (Hg) was analyzed using a Mercury Analyzer (MA-800, Taiwan).
2.4 DNA extraction, PCR amplification and high-throughput sequencing
The total DNA was extracted from 0.3 g of sediment using a FastDNA spin kit (MP Biomedicals, USA). The extracted DNA was examined with 3 μL in 1% agarose gels by electrophoresis. The V3–V4 hypervariable region of the 16S rRNA gene was amplified using primer pairs 338F and 806R. Two different sample-specific barcodes were added to the forward primer to reduce the barcode-specific bias.32 Each sample was amplified with a 20 μL reaction system using the following protocol: 95 °C for 3 min, 27 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, and a final extension at 72 °C for 10 min. All DNA samples were sent to Shanghai Majorbio for Illumina MiSeq sequencing. The raw data have been submitted to the National Center for Biotechnology Information (NCBI) under the accession number SRP113571.2.5 Statistical analyses
The sequencing data were analyzed based on the Mothur software package and SILVA database.33,34 To guarantee the quality of subsequent analysis, sequences with incorrect matching or a length less than 50 bp and reads with low quality, should be discarded in the overlapped area. The paired-end reads were paired-aligned to form tagged barcode sequences by overlapped reads. Universal primer sequences were discarded after assembling the tagged barcode sequences for further analysis.35 The remaining clear tagged barcode sequences, which failed to finish the matches according to the comparison with reference sequences from SILVA database, were removed.A clustering threshold of 97% similarity was adopted to establish an operational taxonomic unit (OTU) table in Mothur.33 The α-diversity indices of each sample were calculated using the vegan package in R (v. 3.12; http://www.r-project.org/). Community analysis was conducted based on the phylum and genus level, and normalization of each sample was carried out prior to comparison. The relative abundances of the communities were analyzed using the R package gplots.36 The relationships between the bacterial community, urbanization index and environmental variables, were analyzed using SPSS 20.0. Cluster analysis was carried out using the software PAST, which is based on Bray–Curtis distances, to illuminate the similarity between different samples. A redundancy analysis (RDA) was adopted on the condition that the correlations between the distribution of the bacterial community and both the urbanization and environmental variables are significant for exploring how urban agglomeration affects the ecological aquatic environment. The metabolic function of the active-microbiome based on the genus level was predicted via the METAGENassist database.37
3. Results
3.1 Assessment of urbanization level
Different weights were calculated based on various indices which formed the comprehensive urbanization index system (Table 1). In the first grade, the economic aspect made the largest contribution (around 41.8%) to urbanization along the Yangtze River, followed by spatial urbanization (35.7%), demographic urbanization (12.4%) and social urbanization (10.1%). Economic urbanization and spatial urbanization occupied major positions (77.5%) in the development of urbanization. In the basic grade index, the number of built-up areas per capita (21.1%) and GDP3 (19.8%) constituted the two heaviest weighted of the eight indicators, followed by the number of built-up areas (14.6%) and the PCGDP (12.2%).Table 2 shows the comprehensive urbanization levels and signal index values of all of these cities. Among the 12 target sites, the value of the urbanization level fluctuated between 0.065 and 0.611, and three thresholds of the 25th, 50th and 75th percentiles of the urbanization level values (ULV) were 0.202, 0.338 and 0.475, respectively (Fig. S1, ESI†). Thus, the urbanization level can be classified into four groups: low urbanization level (ULV ≤ 0.202), lower middle urbanization level (0.202 < ULV < 0.338), upper middle urbanization level (0.338 < ULV < 0.475) and high urbanization level (ULV ≥ 0.475).
3.2 Variability in the diversity and composition of the bacterial community
A total of 816![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873 sequences were generated by 16S rRNA MiSeq sequencing among all the sampling sites both in summer and in winter, of which a 3% cut-off level based on the OTU numbers and the diversity indices was adopted (Table S1, ESI†). Rarefaction analysis indicated that the bacterial diversity in sediments detected were generally high (Fig. S2, ESI†). The OTU numbers of 24 samples were proved with a range of 461 (Xuliujing (XL), in winter) to 2360 (Maanshan (MA), in winter). Bacterial α-diversity indices showed the same decreasing trends both in summer and in winter except the sample WXsu (Fig. S3, ESI†).
873 sequences were generated by 16S rRNA MiSeq sequencing among all the sampling sites both in summer and in winter, of which a 3% cut-off level based on the OTU numbers and the diversity indices was adopted (Table S1, ESI†). Rarefaction analysis indicated that the bacterial diversity in sediments detected were generally high (Fig. S2, ESI†). The OTU numbers of 24 samples were proved with a range of 461 (Xuliujing (XL), in winter) to 2360 (Maanshan (MA), in winter). Bacterial α-diversity indices showed the same decreasing trends both in summer and in winter except the sample WXsu (Fig. S3, ESI†).
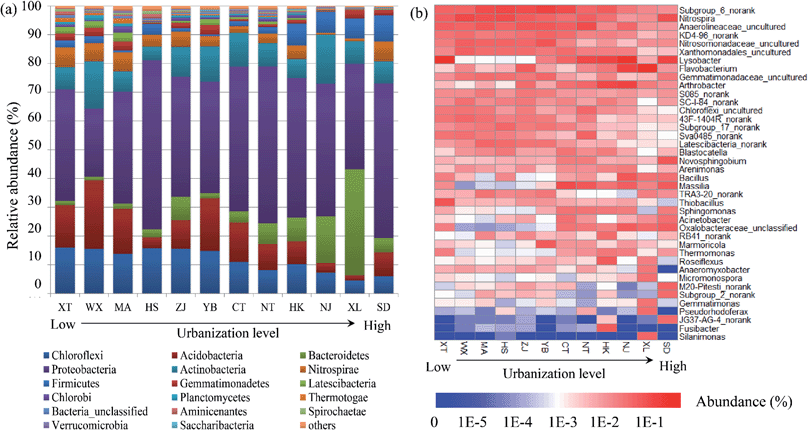
The bacterial community compositions were analyzed at the phylum level and genus level respectively based on normalizing the library size to 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 sequences both in summer and winter. Mean values of the bacterial community compositions based on two seasons were calculated for ultimate analysis. The top 10 bacterial phyla and four classes of Proteobacteria were selected for relative abundance analysis (Fig. 2a). Owing to the low abundance, Epsilonproteobacteria (<1%) was classified to a cluster called ‘others’ with other remaining sequences. Proteobacteria (averaging 44.2%), Chloroflexi (averaging 11.5%), Acidobacteria (averaging 10.8%), Actinobacteria (averaging 9.8%) and Bacteroidetes (averaging 7.9%) were the top five dominant phyla. Regarding the Proteobacteria, Betaproteobacteria (averaging 14.8% abundance) was the most dominant subdivision, followed by the Gammaproteobacteria (averaging 12.7% abundance), Alphaproteobacteria (averaging 8.8% abundance) and Deltaproteobacteria (averaging 7.7% abundance). The relative abundances of different genera are shown in Fig. 2b. Subgrouup_6_norank (Acidobacteria), Nitrospira (Nitrospirae) and Anaerolineaceae_uncultured (Chloroflexi) were the top three genera.
455 sequences both in summer and winter. Mean values of the bacterial community compositions based on two seasons were calculated for ultimate analysis. The top 10 bacterial phyla and four classes of Proteobacteria were selected for relative abundance analysis (Fig. 2a). Owing to the low abundance, Epsilonproteobacteria (<1%) was classified to a cluster called ‘others’ with other remaining sequences. Proteobacteria (averaging 44.2%), Chloroflexi (averaging 11.5%), Acidobacteria (averaging 10.8%), Actinobacteria (averaging 9.8%) and Bacteroidetes (averaging 7.9%) were the top five dominant phyla. Regarding the Proteobacteria, Betaproteobacteria (averaging 14.8% abundance) was the most dominant subdivision, followed by the Gammaproteobacteria (averaging 12.7% abundance), Alphaproteobacteria (averaging 8.8% abundance) and Deltaproteobacteria (averaging 7.7% abundance). The relative abundances of different genera are shown in Fig. 2b. Subgrouup_6_norank (Acidobacteria), Nitrospira (Nitrospirae) and Anaerolineaceae_uncultured (Chloroflexi) were the top three genera.
3.3 Response of bacterial diversity and composition to the urbanization levels
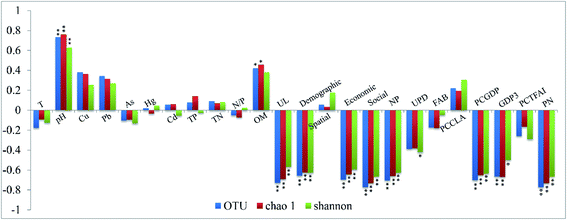
The physiochemical properties of the aquatic environment and the collected sediment are summarized in Table S2 in the ESI.† The sediments of the Yangtze River are slightly alkaline and decreasing trends of pH and organic matter content were observed close to the estuary of the Yangtze River.In order to reveal the relevance between the bacterial α-diversity and urbanization indices or environmental variables, and to uncover which grade of the urbanization level would significantly influence the variations in the bacterial diversity, a Pearson correlation analysis was conducted between the bacterial diversity and urbanization indices (Fig. 3). In general, the diversities in the bacterial community in the sediment of the Yangtze River were significantly correlated with the urbanization level (all correlation coefficients of <−0.56, P < 0.01), which was also confirmed in Fig. S4.† In the first grade index, the demographic aspect (all correlation coefficients of <−0.62, P < 0.01), the economic aspect (all correlation coefficients of <−0.59, P < 0.01) and social aspect (all correlation coefficients of <−0.66, P < 0.01) played significant roles in the variation of the bacterial diversities. Based on the basic grade index, the percentage of the nonagricultural population (PNP) (all correlation coefficients of <−0.62, P < 0.01), PCGDP (all correlation coefficients of <−0.63, P < 0.01) and number of phones per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people (NP) (all correlation coefficients of <−0.66, P < 0.01) were significantly related to the bacterial diversity, followed by the GDP3 (all correlation coefficients of <−0.5, P < 0.05). Regarding the physicochemical variables, the pH (all correlation coefficients of >0.63, P < 0.01) was significantly correlated with the bacterial diversity, followed by the organic matter content.
000 people (NP) (all correlation coefficients of <−0.66, P < 0.01) were significantly related to the bacterial diversity, followed by the GDP3 (all correlation coefficients of <−0.5, P < 0.05). Regarding the physicochemical variables, the pH (all correlation coefficients of >0.63, P < 0.01) was significantly correlated with the bacterial diversity, followed by the organic matter content.
In addition to the bacterial diversity, the bacterial community compositions could also have remarkable impacts on the river ecological environment. Furthermore, how the bacterial community compositions responded to the urbanization level in the sediment of the Yangtze River is still ambiguous. Therefore, cluster analysis and redundancy analysis were applied to further explore the correlation between the bacterial community compositions and the urbanization level.
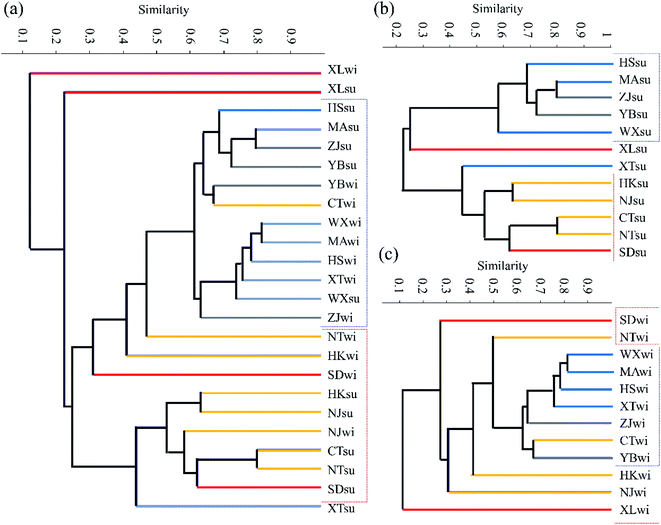
Fig. 4 shows the clustering conditions of the bacterial community composition based on the Bray–Curtis similarity metric using cluster analysis. The results showed that similarities between the bacterial communities exhibited obvious relevance to the urbanization level. The lower the urbanization levels, the higher the similarities in the bacterial community compositions observed. Moreover, the degree of similarity between samples also decreased with the rise in the urbanization level. In addition, an obviously enhanced relative abundance of Bacteroidetes was observed at the XL site compared to the other sites. Bacteroidetes are also a type of anaerobic bacterium, which often live in human or animal intestines. With the higher demographic aspect values of XL, it could be inferred that domestic water containing lots of Bacteroidetes was discharged into urban rivers of XL, and these tributaries flew into the Yangtze River, making a high proportion of Bacteroidetes at the XL site.
Among all of the environmental variables in the RDA shown in Fig. 5, the PCGDP (13.9%, P < 0.01) was found to be most significantly related to the changes in the bacterial community compositions, followed by the temperature (10.8%, P < 0.01) and GDP3 (8.4%, P < 0.05). Both the bacterial diversity and community compositions were significantly correlated with the PCGDP (P < 0.01), followed by the GDP3 (P < 0.05). PCGDP and GDP3 showed positive relationships with the population composition of bacteria at sites XL, Shidongkou (SD), and Nanjing (NJ), which were proved at the high urbanization level. In addition, the PCGDP and GDP3 were observed to be significantly related to the pH value (Table S3, ESI†). Thus, another RDA was conducted between the bacterial community and the physicochemical parameters to illuminate whether pH was significantly correlated to the community compositions. The results also revealed that the pH (P < 0.05) was significantly related to the bacterial community (Fig. S5, ESI†).
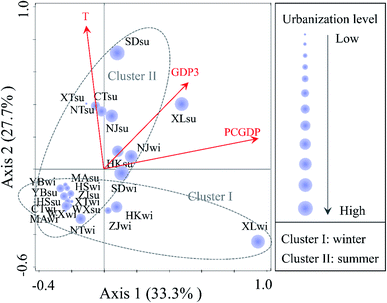 | ||
| Fig. 5 RDA biplot of the bacterial community composition and urbanization index, as well as the environmental variables. Only significant variables (P < 0.05) are shown in the figure. The representations of T, GDP3 and PCGDP correspond with the meanings for the same variables shown in Fig. 3. The size of the purple circle indicates the level of urbanization. | ||
To assess the linkage between individual bacterial genera and the urbanization level, correlation analysis was applied using SPSS 20.0 (Table S4, ESI†). Among all genera, only three (Oxalobacteraceae_unclassified, Xanthomonadales_uncultured and 43F-1404R_norank) were significantly correlated with the urbanization level. Results revealed that Oxalobacteraceae_unclassified (Betaproteobacteria) (P < 0.01) showed a significantly positive relationship between the bacterial abundance and the urbanization level, while Xanthomonadales_uncultured (Gammaproteobacteria) (P < 0.01) and 43F-1404R_norank (Deltaproteobacteria) (P < 0.01) significantly decreased with an elevated urbanization level (Fig. S6, ESI†).
3.4 Potential metabolic characteristics in the bacterial community influenced by different urbanization levels
To evaluate the possible functional features of bacterial communities along the urbanization level gradient, the metabolic function of the microbiome (Fig. 6a) based on the OTU level was predicted via the METAGENassist database.37 Among all of the bacterial metabolic functions, the ammonia oxidizer (42.88% on average), sulfate reducer (33.15% on average), dehalogenation (30% on average), xylan degrader (20.98% on average), nitrite reducer (20.8% on average) and sulfide oxidizer (20.2% on average) were the most dominant functions of the microbiome. Dinitrogen-fixing (0.1%) and gramicidin producer (0.2%) were only found in samples with a high urbanization level, while cellobiose degrader and biomass degrader were absent in these samples.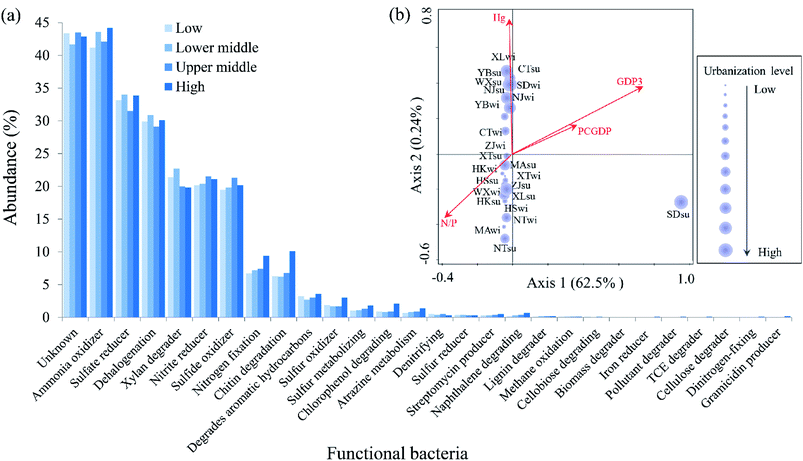 | ||
| Fig. 6 Comparison of the metabolic groups in bacterial communities. (a) Variation in the functional bacteria along the urbanization level gradient according to the METAGENassist analysis and (b) relationship between the metabolic bacteria and significant influenced variables according to the RDA analysis were observed. The meanings of GDP3, PCGDP and N/P correspond with the interpretation of the same variable in Fig. 3. The size of the purple circle indicates the level of urbanization. | ||
The RDA analysis and correlation analysis were conducted to assess the relationships between the metabolic functional bacteria and urbanization index, as well as the environmental variables. Results based on the RDA analysis (Fig. 6b) showed that the GDP3 (35.9%, P < 0.05), N/P (12.9, P < 0.05) and PCGDP (8.8%, P < 0.05) were significantly correlated with variations of the metabolic bacteria. No significant seasonal difference was observed based on the metabolic bacteria compared to the total bacteria. On one hand, two economic urbanization indices GDP3 and PCGDP were correlated with the formation of compositions of functional bacteria and the latter was significantly related to the pH (P < 0.01), Cu (P < 0.05) and Pb (P < 0.05) in Table S3.†
4. Discussion
The urbanization level of the different cities along the Yangtze River was investigated through an urbanization system which consists of the demographic, spatial, economic, and social aspects. Among the cities located at the Yangtze River estuary, two cities belonged to a high urbanization level while the other four belonged to a lower level. The urbanization level along the Yangtze River increased with decreasing distance to the Yangtze River estuary (Fig. S1†), which indicated the higher urbanization level of the Yangtze River Delta among the three largest national urban agglomerations.38 Economic urbanization was the dominant aspect influencing the urbanization along the river (41.8%) and showed significant correlations with the bacterial communities, which was inconsistent with some previous studies. Zhao et al.30 found that the economic and social aspects made dominant contributions to the urban development. While Li et al.29 discovered that the social and demographic aspects were the most important. This difference could be attributed to the different processes of urbanization. Cities in the Yangtze River Delta have entered the middle and advanced state, identified with economic development, while others are still characterized by spatial expansion. Thus, the spatial aspects (e.g., land use) and the economic aspect (e.g., GDP3) were the most important factors, and were related to the resources and energy consumption of cities along the Yangtze River.16,17The composition of the bacterial communities in the sediment of the Yangtze River was analyzed at the phyla level. Of all of the found phyla, a clear decreasing tendency of the Chloroflexi and Acidobacteria and a significant increase in the relative abundance of Bacteroidetes was observed along an elevated urbanization level gradient, while Proteobacteria was relatively constrained with insignificant changes in spite of the highest occupancy (Fig. 2). This result may be explained by the high adaptability of Proteobacteria in various discrepant ecological habitats such as in freshwater sediment and sludge samples,33 and the same environmental adaptability of Betaproteobacteria was also observed.36,45 In particular, Bacteroidetes were accustomed to rivers polluted by various masses, owing to the strong ability of Bacteroidetes to degrade high-molecular-weight organic matter.46 In addition, the abundance of genus Nitrospira was testified to be dominant and this genus was significantly correlated with the N-cycle in a previous study.36
The diversity of bacterial communities was also investigated based on the abundant table of OTU. Bacterial diversity showed a significant decline (P < 0.01) along the urbanization level. The lower bacterial diversity at the estuary of the Yangtze River may be related to the discharge of complicated wastewater at the Yangtze River Delta, which was inextricably correlated to the high urbanization level.1,39 Additionally, the hypothesis of the seawater back flow and the bacteria that have a low tolerance to salinity must be taken into account as a covariate to explain the decrease in the bacterial α-diversity.40 Meanwhile, social urbanization was most significantly correlated with the variations of the bacterial diversity, followed by the economic urbanization. Both the GDP3 and PCGDP (P < 0.05) were observed to be significantly correlated with the variations of the bacterial community and the metabolic bacteria. The synergistic effect of both aspects would significantly influence the eco-environment, as the positive linkage between the expansion rate of the socio-economic scale and the eco-environmental stress was verified.30 It is generally accepted that the social consumption level was associated with the economic development. The economic urbanization, related to the PCGDP and GDP3, was correlated with the development of the secondary and the tertiary industry and would cause a great deal of industrial and domestic pollution. Finally, huge ecological stress derived from external disturbances would destroy the biological diversity and the compositions of communities.
It was proved that the urbanization could affect microorganisms by changing soil properties and biotope conditions.47 A previous study also showed that animal communities were correlated with the urban development and that minimal diversity was observed in the most urbanized areas.48 Utz et al.15 analyzed the response of the physicochemical variables to urbanization in rivers, and found that the properties of streams were substantially altered by urbanization, consequently resulting in the loss of biodiversity and a decline in function of the ecosystem. Taking no account of the dominant influence of urbanization indices, among the physicochemical variables, the impact of pH was undoubtedly vital to the variations of bacterial diversity and community, followed by organic matter and N/P. To the best of our knowledge, the pH in the sediment is easily disturbed by acidic or alkaline pollutants derived from anthropogenic activities such as urbanization and further affects the bacterial community. Organic matter as a kind of important allochthonous resource has been proved to have a dominant role in forming bacterial communities. Cotano and Villate et al.49 reported that organic matter distribution was influenced by anthropogenic activities, such as the discharge of sewage. Finally, organic matter in rivers shapes the microbial communities.50 In addition, Zhi et al.51 also found that the abundance of the amoA gene was related to the pH, NH4+ and NO3−.
Regarding the variation of the typical bacteria, the interaction between the ‘mass effects’ and ‘species sorting’ based on the meta-community concept should be taken into account.52 Bacteria were reported to transport passively into rivers because streams were regarded as a significant part of the hydrological cycle. Under anthropogenic influence such as urbanization, receiving water bodies would respond more quickly to the ‘mass effect’ of dispersing microorganisms compared to the rate of ‘species sorting’ based on the concept of river continuum. Thus, a secondary succession of an established community caused by external disturbance is reasonable.10 It is worth noting that the ‘mass effect’ related to urbanization through the influence of land use and industrialization development could explain the variations in biodiversity.53 The higher heterogeneity of bacterial community compositions along the elevated urbanization level may be related to more external disturbances, consistent with some previous results. Stressful streams caused by watershed urbanization receive severe and frequent physicochemical disturbance and hence alter the bacterial community composition in streams.39,41 In addition, previous studies also illuminated the linkage between variations in the bacterial communities and the urbanization development.39 In this investigation, the ‘mass effect’ was mainly reflected through the variations of the sediment pH as only the pH is significantly correlated with the urbanization index (Table S3, ESI†) and therefore ultimately influenced the bacterial community diversities. Also, only the pH is significantly correlated with the organic matter amongst the other physicochemical parameters as shown in Table S5 in the ESI†. Thus, the ‘mass effect’, which was related to urbanization, was significantly correlated to the variations in the bacterial compositions in the sediment of the Yangtze River. As shown in Fig. 2, some taxa were identified as dominant taxa. Peter et al.42 indicated that the Betaproteobacteria, which has a strong adaptability to complicated environments, occurred in turbid streams, but were absent in clear lakes. In addition, Oxalobacteraceae was generally recognized as fast-growing organisms.43 The decreasing trend of Xanthomonadales_uncultured (Gammaproteobacteria) along the elevated pollution gradient was in agreement with the variation tendencies reported in a previous study,22 while Deltaproteobacteria showed a different variation trend. The different patterns of bacterial communities verified the existence of deviating the assembly mechanisms in different ecosystem.
Nine main metabolic functions were further analyzed under the effect of the urbanization level in an attempt to reveal how the dominant bacterial function (abundance > 20%) changed along with the urbanization level (Fig. S7, ESI†). The metabolic activities of bacteria along four urbanization levels show slight increasing trends except for the xylan degrader, indicating that the urbanization level slightly accelerated the formation of the bacterial metabolic function. The xylan degrader was the only function observed to decrease along the urbanization level gradient. Significant increasing trends of nitrogen fixation and chitin degradation were observed. In addition, the nitrogen fixation and chitin degradation in the high urbanization level were also significantly higher than the other urbanization levels. Thus, the rapid development would result in the targeted development of dominant functional bacteria and ultimately cause species simplification. The metabolic function of the bacteria related to the N-cycle and S-cycle played important roles in the sediment of the Yangtze River. The N-cycle is significant to the biogeochemical cycle and is easily disturbed by input of nutrients.44 Functional bacteria related to the S-cycle verified the importance of regulating the metal-rich extreme environments in a previous study.37 The results revealed that some metabolic functions such as the ammonia oxidizer, dehalogenation and xylan degrader displayed significantly decreasing trends in NT (ULV = 0.39), which may be correlated to the industrial patterns of NT, for which the textile industry is very advanced. The abrupt discharge of textile-dyeing wastewater with high contents of organic pollutant such as aromatic amines54 would inhibit the metabolic function of the sediment bacteria. Moreover, the abundance of nitrogen fixation, chitin degradation and the degradation of aromatic hydrocarbons displayed increasing trends along with the urbanization level, especially in the variation of the abundance of nitrogen fixation. It is worth noting that nitrogen-fixing bacteria were attracted to the habitat containing organotrophs which significantly increases along with the urbanization level.
The meta-community concept related to the interactions between the ‘mass effect’ and ‘species sorting’ was also used to explain the ecological succession of the bacterial metabolic function according to previous investigations. The deteriorative physicochemical signatures of river ecology that resulted from rapid urbanization could severely restrain the activity of the microflora during various forms of metabolism, for instance, the N-cycle, S-cycle and sugar metabolism.37 The influence of the mass effect, reflected by the environmental variables on the bacterial community, would exceed species sorting when a strong external disturbance exists and species sorting would be a candidate mechanism contributing to formation of the metacommunity.55 The transition between bacterial communities under the influence of the allothogenic mass can be explained by two processes: migration and survival of the fittest. On the one hand, based on the dispersal-based concepts, the bacteria were transported passively seeking the most suitable habitat under the input of complicated pollutants. On the other hand, the rule of those that are naturalized will live can also be applied to explain the changes in the microcommunity. Species selection based on interspecific competition rather than stochastic immigration, thus, played a significant role in the changing community assembly and is therefore a reasonable assumption.10,55 As a previous study revealed, both domestic and industrial pollutants, which are highly correlated with the development of the secondary and the tertiary industry, would influence the bacterial community.44 On the other hand, the urbanization index GDP3 and the physicochemical parameters, such as pH and organic matter, were observed to be correlated with variations in the majority of the metabolic bacteria (Table S8, ESI†). This result also highlighted the vital influence of the tertiary industry, as well as the mass effect caused by economic development on the bacterial community.
The results of this investigation strongly suggest that an attempt to improve the protection of the Yangtze River, controlling the pollution derived from tertiary industry entering into the Yangtze River is of great importance, owing to the huge impact of the tertiary industry on the eco-environment of the Yangtze River Economic Zone, followed by the secondary industry. The findings in this investigation provide an important basis for future work to control and improve the water quality in the Yangtze River, which is undergoing rapid urban development.
Funding information
This work was supported by the National Natural Science Foundation of China [grant numbers 51779076]; the Foundation for Innovative Research Groups of the National Natural Science Foundation of China [grant number 51421006]; the Fundamental Research Funds for the Central Universities [grant number 2019B52514]; the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); the Six Talent Peaks Project in Jiangsu Province [grant number 2016-JNHB-007]; the 333 High Level Talents training Project of Jiangsu Province the National Natural Science Foundation of China [grant number 51509072] and the Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.Conflicts of interest
The authors declare that they have no competing interests.References
- E. R. Moffett, K. S. Simon and J. S. Harding, Urbanisation and earthquake disturbance influence microbial nutrient limitation in streams, Freshwater Biol., 2015, 60, 1671–1687 CrossRef CAS.
- X. Zhao, Z. Wei, Y. Zhao, B. Xi, X. Wang, T. Zhao, X. Zhang and Y. Wei, Environmental factors influencing the distribution of ammonifying and denitrifying bacteria and water qualities in 10 lakes and reservoirs of the Northeast, China, Microb. Biotechnol., 2015, 8, 541–548 CrossRef CAS PubMed.
- A. T. Poret-Peterson, B. Ji, E. Engelhaupt and J. Gulledge, Soil microbial biomass along a hydrologic gradient in a subsiding coastal bottomland forest: implications for future subsidence and sea-level rise, Soil Biol. Biochem., 2007, 39, 641–645 CrossRef CAS.
- Z. Yu, J. Yang, S. Amalfitano, X. Yu and L. Liu, Effects of water stratification and mixing on microbial community structure in a subtropical deep reservoir, Sci. Rep., 2014, 4, 5821 CrossRef CAS PubMed.
- J. Harris, Soil Microbial Communities and Restoration Ecolog Facilitators or Followers?, Science, 2009, 325, 573–574 CrossRef CAS PubMed.
- X. Qin, G. Huang, B. Chen and B. Zhang, An interval-parameter waste-load-allocation model for river water quality management under uncertainty, Environ. Manag., 2009, 43, 999–1012 CrossRef PubMed.
- W. H. Hartman, C. J. Richardson, R. Vilgalys and G. L. Bruland, Environmental and anthropogenic controls over bacterial communities in wetland soils, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17842–17847 CrossRef CAS PubMed.
- W. Mhuantong, S. Wongwilaiwalin, T. Laothanachareon, L. Eurwilaichitr, S. Tangphatsornruang, B. Boonchayaanant, T. Limpiyakorn, K. Pattaragulwanit, T. Punmatharith, J. McEvoy, E. Khan, M. Rachakornkij and V. Champreda, Survey of Microbial Diversity in Flood Areas during Thailand 2011 Flood Crisis Using High-Throughput Tagged Amplicon Pyrosequencing, PLoS One, 2015, 10, e0128043 CrossRef PubMed.
- V. Torsvik, L. Ovreas and T. Thingstad, Prokaryotic Diversity-Magnitude, dynamics, and controlling factors, Science, 2002, 296, 1064–1066 CrossRef CAS PubMed.
- D. Savio, L. Sinclair, U. Z. Ijaz, J. Parajka, G. H. Reischer, P. Stadler, A. P. Blaschke, G. Bloschl, R. L. Mach, A. K. Kirschner, A. H. Farnleitner and A. Eiler, Bacterial diversity along a 2600 km river continuum, Environ. Microbiol., 2015, 17, 4994–5007 CrossRef CAS PubMed.
- J. B. Cotner and B. A. Biddanda, Small Players, Large Role: Microbial Influence on Biogeochemical Processes in Pelagic Aquatic Ecosystems, Ecosystems, 2002, 5, 105–121 CrossRef CAS.
- L. H. Zeglin, Stream microbial diversity in response to environmental changes: review and synthesis of existing research, Front. Microbiol., 2015, 6, 454 Search PubMed.
- V. Thiyagarajan, M. M. Tsoi, W. Zhang and P. Y. Qian, Temporal variation of coastal surface sediment bacterial communities along an environmental pollution gradient, Mar. Environ. Res., 2010, 70, 56–64 CrossRef CAS PubMed.
- R. Wagner, D. Zona, W. Oechel and D. Lipson, Microbial community structure and soil pH correspond to methane production in Arctic Alaska soils, Environ. Microbiol., 2017, 19, 3398–3410 CrossRef CAS PubMed.
- R. M. Utz, K. N. Eshleman and R. H. Hilderbrand, Variation in physicochemical responses to urbanization in streams between two Mid-Atlantic physiographic regions, Ecol. Appl., 2011, 21, 402–415 CrossRef PubMed.
- Q. Gu, H. Wang, Y. Zheng, J. Zhu and X. Li, Ecological footprint analysis for urban agglomeration sustainability in the middle stream of the Yangtze River, Ecol. Model., 2015, 318, 86–99 CrossRef.
- L. Qu, T. Zhang and S. Liang, Waste management of urban agglomeration on a life cycle basis, Resour. Conserv. Recycl., 2013, 78, 47–53 CrossRef.
- P. J. Meffert and F. Dziock, What determines occurrence of threatened bird species on urban wastelands?, Biol. Conserv., 2012, 153, 87–96 CrossRef.
- L. Zhang, Z. Zhang, Y. Chen and Y. Fu, Sediment characteristics, floods, and heavy metal pollution recorded in an overbank core from the lower reaches of the Yangtze River, Environ. Earth Sci., 2015, 74, 7451–7465 CrossRef CAS.
- S. Yin, C. Feng, Y. Li, L. Yin and Z. Shen, Heavy metal pollution in the surface water of the Yangtze Estuary: a 5-year follow-up study, Chemosphere, 2015, 138, 718–725 CrossRef CAS PubMed.
- L. Cao, G. H. Hong and S. Liu, Heavy metal pollution in the surface water of the Yangtze Estuary: a 5-year follow-up study, Mar. Pollut. Bull., 2015, 95, 458–468 CrossRef CAS PubMed.
- J. Xiong, X. Ye, K. Wang, H. Chen, C. Hu, J. Zhu and D. Zhang, Biogeography of the sediment bacterial community responds to a nitrogen pollution gradient in the East China Sea, Appl. Environ. Microbiol., 2014, 80, 1919–1925 CrossRef PubMed.
- T. Jiang, Z. Yu, X. Song and X. Cao, Nitrogen budget in the Changjiang River drainage area, Chin. J. Oceanol. Limnol., 2012, 30, 654–667 CrossRef CAS.
- Y. Zhang, X. Xie, N. Jiao, S. S. Y. Hsiao and S. J. Kao, Diversity and distribution of amoA-type nitrifying and nirS-type denitrifying microbial communities in the Yangtze River estuary, Biogeosciences, 2014, 11, 2131–2145 CrossRef.
- Y. Chen, Y. Zhen, H. He, X. Lu, T. Mi and Z. Yu, Diversity, abundance, and spatial distribution of ammonia-oxidizing beta-proteobacteria in sediments from Changjiang Estuary and its adjacent area in East China Sea, Microb. Ecol., 2014, 67, 788–803 CrossRef CAS PubMed.
- P. López-García, F. Rodríguez-Valera, C. Pedrós-Alió and D. Moreira, Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton, Nature, 2001, 409, 603–607 CrossRef PubMed.
- S. Y. D. W. Moon-van der Staay and D. Vaulot, Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity, Nature, 2001, 409, 607–610 CrossRef CAS PubMed.
- C. v. M. Susannah Green Tringe, A. Kobayashi, A. A. Salamov, K. Chen, H. W. Chang, M. Podar, M. Jay, E. J. M. Short, J. C. Detter, B. Peer, P. Hugenholtz and E. M. Rubin, Comparative Metagenomics of Microbial Communities, Science, 2005, 308, 554–557 CrossRef PubMed.
- Y. Li, Y. Li, Y. Zhou, Y. Shi and X. Zhu, On the relationship between landscape ecological patterns and water quality across gradient zones of rapid urbanization in coastal China, J. Environ. Manage., 2012, 98, 127–133 CrossRef PubMed.
- Y. Zhao, S. Wang and C. Zhou, Understanding the relation between urbanization and the eco-environment in China's Yangtze River Delta using an improved EKC model and coupling analysis, Sci. Total Environ., 2016, 571, 862–875 CrossRef CAS PubMed.
- A. Zoppini, S. Amalfitano, S. Fazi and A. Puddu, Dynamics of a benthic microbial community in a riverine environment subject to hydrological fluctuations (Mulargia River, Italy), Hydrobiologia, 2010, 657, 37–51 CrossRef CAS.
- L. Wilhelm, K. Besemer, L. Fragner, H. Peter, W. Weckwerth and T. J. Battin, Altitudinal patterns of diversity and functional traits of metabolically active microorganisms in stream biofilms, ISME J., 2015, 9, 2454–2464 CrossRef CAS PubMed.
- S. Liu, H. Ren, L. Shen, L. Lou, G. Tian, P. Zheng and B. Hu, pH levels drive bacterial community structure in sediments of the Qiantang River as determined by 454 pyrosequencing, Front. Microbiol., 2015, 6, 285 Search PubMed.
- P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn and C. F. Weber, Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol., 2009, 75, 7537–7541 CrossRef CAS PubMed.
- H. W. Zhou, D. F. Li, N. F. Tam, X. T. Jiang, H. Zhang, H. F. Sheng, J. Qin, X. Liu and F. Zou, BIPES, a cost-effective high-throughput method for assessing microbial diversity, ISME J., 2011, 5, 741–749 CrossRef CAS PubMed.
- L. Niu, Y. Li, P. Wang, W. Zhang, C. Wang and Q. Wang, Understanding the linkage between elevation and the activated-sludge bacterial community along a 3,600-meter elevation gradient in China, Appl. Environ. Microbiol., 2015, 81, 6567–6576 CrossRef CAS PubMed.
- M. Fan, Y. Lin, H. Huo, Y. Liu, L. Zhao, E. Wang, W. Chen and G. Wei, Microbial communities in riparian soils of a settling pond for mine drainage treatment, Water Res., 2016, 96, 198–207 CrossRef CAS PubMed.
- G. Tian, J. Jiang, Z. Yang and Y. Zhang, The urban growth, size distribution and spatio-temporal dynamic pattern of the Yangtze River Delta megalopolitan region, China, Ecol. Model., 2011, 222, 865–878 CrossRef.
- S. E. B. Wang Si-Yi, D. Wallenstein Matthew, P. Wright Justin and S. Bernhardt Emily, Watershed Urbanization Alters the Composition and Function of Stream Bacterial Communities, PLoS One, 2011, 6, e22972 CrossRef PubMed.
- Y. Hu, L. Wang, Y. Tang, Y. Li, J. Chen, X. Xi, Y. Zhang, X. Fu, J. Wu and Y. Sun, Variability in soil microbial community and activity between coastal and riparian wetlands in the Yangtze River estuary – Potential impacts on carbon sequestration, Soil Biol. Biochem., 2014, 70, 221–228 CrossRef CAS.
- S. D. Allison and J. B. Martiny, Colloquium paper: resistance, resilience, and redundancy in microbial communities, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(suppl. 1), 11512–11519 CrossRef CAS PubMed.
- H. Peter and R. Sommaruga, Shifts in diversity and function of lake bacterial communities upon glacier retreat, ISME J., 2016, 10, 1545–1554 CrossRef CAS PubMed.
- Z. T. Aanderud, S. E. Jones, N. Fierer and J. T. Lennon, Resuscitation of the rare biosphere contributes to pulses of ecosystem activity, Front. Microbiol., 2015, 6, 24 Search PubMed.
- M. Xu, Q. Zhang, C. Xia, Y. Zhong, G. Sun, J. Guo, T. Yuan, J. Zhou and Z. He, Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments, ISME J., 2014, 8, 1932–1944 CrossRef CAS PubMed.
- E. S. Lindstrom, M. P. Kamst-Van Agterveld and G. Zwart, Distribution of Typical Freshwater Bacterial Groups Is Associated with pH, Temperature, and Lake Water Retention Time, Appl. Environ. Microbiol., 2005, 71, 8201–8206 CrossRef PubMed.
- Q. Ye, Y. Wu, Z. Zhu, X. Wang, Z. Li and J. Zhang, Bacterial diversity in the surface sediments of the hypoxic zone near the Changjiang Estuary and in the East China Sea, MicrobiologyOpen, 2016, 5, 323–339 CrossRef CAS PubMed.
- A. T. Reese, A. Savage, E. Youngsteadt, K. L. McGuire, A. Koling, O. Watkins, S. D. Frank and R. R. Dunn, Urban stress is associated with variation in microbial species composition-but not richness-in Manhattan, ISME J., 2016, 10, 751–760 CrossRef PubMed.
- M. Saito and F. Koike, Distribution of wild mammal assemblages along an urban-rural-forest landscape gradient in warm-temperate East Asia, PLoS One, 2013, 8, e65464 CrossRef CAS PubMed.
- U. Cotano and F. Villate, Anthropogenic influence on the organic fraction of sediments in two contrasting estuaries: a biochemical approach, Mar. Pollut. Bull., 2006, 52, 404–414 CrossRef CAS PubMed.
- S. K. Fagervold, S. Bourgeois, A. M. Pruski, F. Charles, P. Kerherve, G. Vetion and P. E. Galand, River organic matter shapes microbial communities in the sediment of the Rhone prodelta, ISME J., 2014, 8, 2327–2338 CrossRef CAS PubMed.
- E. Zhi, Y. Song, L. Duan, H. Yu and J. Peng, Spatial distribution and diversity of microbial community in large-scale constructed wetland of the Liao River Conservation Area, Environ. Earth Sci., 2015, 73, 5085–5094 CrossRef.
- M. A. Leibold, M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau and A. Gonzalez, The metacommunity concept: a framework for multi-scale community ecology, Ecol. Lett., 2004, 7, 601–613 CrossRef.
- Y. Li, Y. Li, S. Qureshi, M. Kappas and K. Hubacek, On the relationship between landscape ecological patterns and water quality across gradient zones of rapid urbanization in coastal China, Ecol. Model., 2015, 318, 100–108 CrossRef CAS.
- J. Liang, X. A. Ning, T. An, J. Sun, Y. Zhang and Y. Wang, Degradation of aromatic amines in textile-dyeing sludge by combining the ultrasound technique with potassium permanganate treatment, J. Hazard Mater., 2016, 314, 1–10 CrossRef CAS PubMed.
- L. Wilhelm, G. A. Singer, C. Fasching, T. J. Battin and K. Besemer, Microbial biodiversity in glacier-fed streams, ISME J., 2013, 7, 1651–1660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00399a |
| This journal is © The Royal Society of Chemistry 2020 |