Open Access Article
Open Access ArticleMicrowave-assisted synthesis of 7-azaindoles via iron-catalyzed cyclization of an o-haloaromatic amine with terminal alkynes†
Yi Leabc,
Zhisong Yangb,
Yumei Chenb,
Dongmei Chenb,
Longjia Yan *bc,
Zhenchao Wangbc and
Guiping Ouyang*abc
*bc,
Zhenchao Wangbc and
Guiping Ouyang*abc
aState Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, State-Local Joint Laboratory for Comprehensive Utilization of Biomass, Center for Research and Development of Fine Chemicals, Guizhou University, Guiyang 550025, China
bSchool of Pharmaceutical Sciences, Guizhou University, Guiyang 550025, China. E-mail: ylj1089@163.com; oygp710@163.com
cGuizhou Engineering Laboratory for Synthetic Drugs, Guiyang 550025, China
First published on 2nd December 2019
Abstract
An efficient and practical procedure was developed to prepare 7-azaindole, starting from an o-haloaromatic amine and corresponding terminal alkynes under microwave irradiation and the scope was demonstrated with a number of examples. The valuable features of this procedure included the iron-catalyzed cyclization, short reaction times and convenient operation. Furthermore, iron catalysis is an interesting alternative to homogeneous catalysis for the synthesis of heterocycles.
Introduction
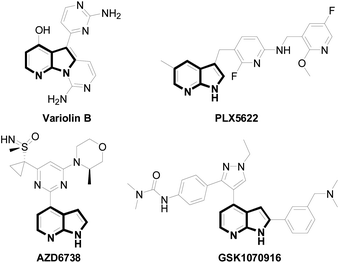
In the recent years, microwave (MW) irradiation in synthetic organic chemistry has attracted considerable practical and theoretical attention as a very effective and non-polluting method of activation.1 Microwave irradiation often helps to reduce reaction times, minimize side products, increase yields and improve reproducibility. Therefore, the technique of microwave-assisted organic synthesis has been widely and successfully employed in dipolar cycloadditions,2 transition metal-catalyzed cross-coupling reactions3 and polymer formation.41H-Pyrrolo[2,3-b]pyridines, often referred to as 7-azaindoles, are bioisosteres of the indole scaffold have been used in diverse areas such as materials and medicinal chemistry due to their physicochemical and pharmacological properties.5 For example, the natural product Variolin B5 in Fig. 1 isolated from an extremely rare antarctic sponge is a promising anti-cancer agent. PLX5622 in Fig. 1,6 a brain pentrant CSF1R inhibitor has been used in Alzheimer's disease (AD). Recently, the 7-azaindole derivatives were also shown potential anti-cancer activity, including AZD6738 a potent and selective ATR kinase inhibitor7 and GSK1070916 an aurora kinase inhibitor.8 Thus, development of synthetic methods to access these 7-azaindoles is of great importance to the drug discovery community.9
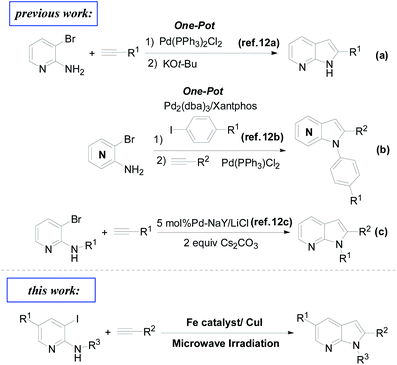
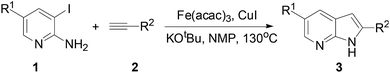
Our group has been focused on metal-catalyzed cross-coupling reactions for the preparation of bioactive heterocycles.10 Based on the broad activities of 7-azaindole, we are interested in developing new strategies to prepare this novel heterocyclic scaffold. In the literature, the common synthetic methods to prepare azaindoles usually start from commercial aminopyridines, followed by Sonogashira alkynylation by subsequent base-mediated cyclization with various base for building up the pyrrole ring.11 As shown in Fig. 2, these methods were always used Pd-catalysts, such as Pd(PPh3)2Cl2,12a Pd2(dba)3,12b and Pd–NaY,12c and also have some disadvantages such as the high price of palladium and problem of pollution. Recently, the establishment of new catalytic methods using iron is attractive owing to the low cost, abundance, ready availability, and very low toxicity of iron.13 Furthermore, due to the availability of several catalytic cycles, iron catalysis offers a potential for orthogonal selectivity when compared to other metals, which allows for streamlined construction of complex molecules by modern cross-coupling chemistry.14 Encouraged by the development of iron-catalytic Sonogashira alkynylation15 and the microwave-assisted synthesis of indole,16 we wish to report the iron-catalyzed cyclization of o-haloaromatic amine with terminal alkynes under the microwave irradiation.
Results and discussion
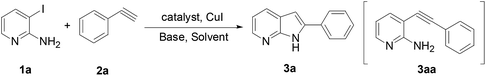
Initially, we chose the commercially available 3-iodo-pyridin-2-ylamine 1a and ethynyl-benzene 2a as model starting materials. We examined the reaction conditions using 10% Pd(PPh3)2Cl2, 10% CuI, 1.5 equiv. of K3PO4 in DMF under microwave irradiation of 100 °C, the reaction proceeded for 30 min and product 3a was obtained in 12%, which was purified the intermediate 3aa for 35% yield (Table 1, entry 1). We subsequently investigated the effect of various catalysts including palladium, silver and iron (Table 1, entries 2–6). Fortunately, we found that Fe(acac)3 was given the best result (Table 1, entry 6). We also tested the amount of CuI, it was no product without CuI and the similar yield using 20% CuI (Table 1, entries 7 and 8). Another blank test was performed only with CuI and shown that 3aa was obtained in 8% yield (Table 1, entry 9). It was no reaction at the absence of Fe(acac)3 and CuI (Table 1, entry 10). Replacement of K3PO4 by KOAc, or K2CO3 led to lower yields and KOtBu gave a satisfactory yield (Table 1, entries 11–13). Next, we screened different organic solvents and identified NMP as the most effective solvent (Table 1, entry 15), while dioxane resulted in a poor yield (Table 1, entry 16). The yield could be further improved by increasing the temperature to a maximum of 130 °C (Table 1, entry 19), whereas higher temperatures led to partial decomposition (Table 1, entry 20). Interestingly, when we used Pd(PPh3)2Cl2 reported by Mueller12a or Pd(dppf)Cl2 under the optimized condition, the yield was 44% and 48%, respectively (Table 1, entries 21 and 22).| Entry | Catalyst | CuI (%) | Base | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reagents and conditions: 1a (1 mmol), 2a (2 mmol), catalyst (0.1 mmol), CuI (0.1 mmol), base (1.5 mmol), solvent (2 mL), 30 min MW, 100 °C.b Isolated yields.c Only 3aa was obtained with 8% yield. | ||||||
| 1 | Pd(PPh3)2Cl2 | 10 | K3PO4 | DMF | 100 | 12 |
| 2 | Pd(dppf)Cl2 | 10 | K3PO4 | DMF | 100 | 21 |
| 3 | AgNO3 | 10 | K3PO4 | DMF | 100 | Trace |
| 4 | AgOAc | 10 | K3PO4 | DMF | 100 | Trace |
| 5 | FeCl3 | 10 | K3PO4 | DMF | 100 | 11 |
| 6 | Fe(acac)3 | 10 | K3PO4 | DMF | 100 | 32 |
| 7 | Fe(acac)3 | — | K3PO4 | DMF | 100 | 0 |
| 8 | Fe(acac)3 | 20 | K3PO4 | DMF | 100 | 31 |
| 9 | — | 10 | K3PO4 | DMF | 100 | —c |
| 10 | — | — | K3PO4 | DMF | 100 | 0 |
| 11 | Fe(acac)3 | 10 | KOAc | DMF | 100 | 28 |
| 12 | Fe(acac)3 | 10 | K2CO3 | DMF | 100 | 25 |
| 13 | Fe(acac)3 | 10 | KOtBu | DMF | 100 | 38 |
| 14 | Fe(acac)3 | 10 | KOtBu | DMSO | 100 | 36 |
| 15 | Fe(acac)3 | 10 | KOtBu | NMP | 100 | 42 |
| 16 | Fe(acac)3 | 10 | KOtBu | Dioxane | 100 | 22 |
| 17 | Fe(acac)3 | 10 | KOtBu | NMP | 110 | 58 |
| 18 | Fe(acac)3 | 10 | KOtBu | NMP | 120 | 61 |
| 19 | Fe(acac)3 | 10 | KOtBu | NMP | 130 | 62 |
| 20 | Fe(acac)3 | 10 | KOtBu | NMP | 140 | 58 |
| 21 | Pd(PPh3)2Cl2 | 10 | KOtBu | NMP | 130 | 44 |
| 22 | Pd(dppf)Cl2 | 10 | KOtBu | NMP | 130 | 48 |
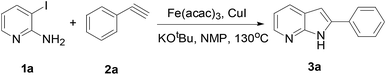
In order to optimize the reaction conditions, we speculated that the equivalent of alkyne and microwave time might also be efficient in promoting the microwave-assisted cyclized reaction. After exploring several reaction conditions (Table 2), 3a was obtained in 72% yield by heating 3 equiv. 2a, 0.1 equiv. Fe(acac)3, 0.1 equiv. CuI, 1.5 equiv. KOtBu in NMP for 60 min at 130 °C (Table 2, entry 4). We also tried the reaction under conventional thermal heating condition and the yield of the desired product was 33% (Table 2, entry 7).
| Entry | 2a (equiv.) | Time (min) | Yieldb (%) |
|---|---|---|---|
| a Reagents and conditions: 1a (1 mmol), 2a, Fe(acac)3 (0.1 mmol), CuI (0.1 mmol), KOtBu (1.5 mmol), NMP (2 mL), MW, 130 °C.b Isolated yields.c Under conventional thermal heating condition. | |||
| 1 | 2 | 30 | 62 |
| 2 | 3 | 30 | 68 |
| 3 | 4 | 30 | 66 |
| 4 | 3 | 60 | 72 |
| 5 | 3 | 90 | 71 |
| 6 | 3 | 120 | 58 |
| 7 | 3 | 120 | 33c |
Having determined the optimal reaction conditions (Table 2, entry 4), the scope of the reaction was explored with different substituted 3-iodo-pyridin-2-ylamine 1 (Table 3), which were prepared by the reaction of pyridin-2-ylamines with Ag2SO4 and I2, to afford various 7-azaindoles 3. As illustrated in Table 3, a variety of substituted 7-azaindoles was readily prepared in good yield through this protocol, and assigned by spectroscopic methods (1H NMR and 13C NMR) and HRMS. Both electron-donating group –CH3 (Table 3, entries 3 and 4) and electron-withdrawing group –CN (Table 3, entries 5–8) of R1 are tolerated at a variety of positions on the aromatic ring. Alkynes bearing benzene ring such as 4-OCH3 and 4-F were also tolerated under reaction conditions as 3b and 3d were afforded in 73% and 68% yields, respectively. Additionally, aliphatic groups afforded the corresponding products with decreased yields (3h). This result was consistent with the observation by Mueller et al. when Pd(PPh3)2Cl2/(1-Ad)2PBn was used as catalyst.12a The introduction of trifluoromethyl group into organic compounds frequently renders remarkable changes in their special size, bioactivity and metabolic stability.17 In this paper, we also show such examples of the CF3 substituted 7-azaindoles (Table 3, entries 9–16). We found that the reaction conditions were adaptable with phenyl (3i) and electron-donating substituents at the aryl alkynes, such as methoxy group (3k). In addition to electron-donating substituent, halogens such as F and Cl were also tolerated (3j, 3m and 3n). However, aryl groups with an electron deficient substituent led to a lower yield (3l). Additionally, aliphatic groups afforded the corresponding products with reduced yields (3o and 3p).
| Entry | R1 | R2 | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reagents and conditions: 1 (1 mmol), 2 (3 mmol), Fe(acac)3 (0.1 mmol), CuI (0.1 mmol), KOtBu (1.5 mmol), NMP (2 mL), 60 min MW, 130 °C.b Isolated yields. | ||||
| 1 | H | Ph | 3a | 72 |
| 2 | H | 4-OCH3-Ph | 3b | 73 |
| 3 | Me | Ph | 3c | 73 |
| 4 | Me | 4-F-Ph | 3d | 68 |
| 5 | CN | Ph | 3e | 65 |
| 6 | CN | 4-F-Ph | 3f | 61 |
| 7 | CN | 4-OCH3-Ph | 3g | 66 |
| 8 | CN | Me | 3h | 43 |
| 9 | CF3 | Ph | 3i | 62 |
| 10 | CF3 | 4-F-Ph | 3j | 56 |
| 11 | CF3 | 4-OCH3-Ph | 3k | 79 |
| 12 | CF3 | 4-CF3-Ph | 3l | 45 |
| 13 | CF3 | 4-Cl-Ph | 3m | 58 |
| 14 | CF3 | 2-Cl-Ph | 3n | 32 |
| 15 | CF3 | Me | 3o | 33 |
| 16 | CF3 | t-Butyl | 3p | 48 |
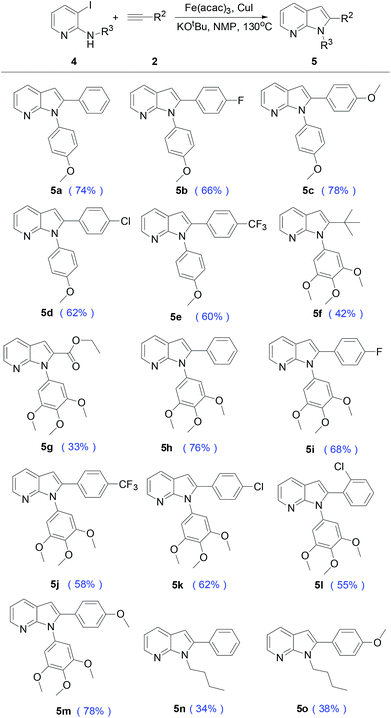
Finally, this procedure was applied to the preparation of 1,2-disubstituted 7-azaindoles 5. As shown in Table 4, the Sonogashira and consequently cyclization could be used for preparation the N-arylation of azaindoles. The electron-donating groups on the phenyl acetylene, such as OCH3 (5c and 5m) were obtained the much higher yield of 78% than the electron-withdrawing group F (5b and 5i), Cl (5d, 5k and 5l) and CF3 (5e and 5j). 3,3,-Dimethyl-but-1-yne led to 2-tert-butyl-1-(3,4,5-trimethoxy-phenyl)-1H-pyrrolo[2,3-b]pyridine 5f (42%), the decrease in yield may be due to decomposition of compound 2 under the MW conditions. Interestingly, the ethyl ester substituted group 5g was also separated with 33% yield under our MW protocol. In addition to substituted aryl group of R3, the alkyl-substituted compound 4 were also compatible with this process, furnishing the desired product 5n and 5o in 34% and 38% yield.
| a Reagents and conditions: 4 (1 mmol), 2 (3 mmol), Fe(acac)3 (0.1 mmol), CuI (0.1 mmol), KOtBu (1.5 mmol), NMP (2 mL), 60 min MW, 130 °C. |
|---|
 |
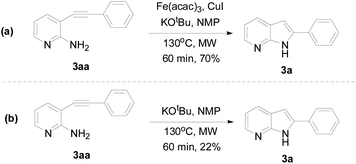
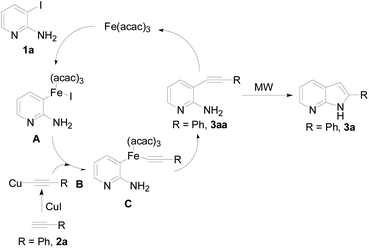
To investigate the mechanism of this transformation, experiments were carried out. The desired product 3a was obtained in 70% yield under standardized reaction conditions (Scheme 1). We also tested the absence of Fe(acac)3 and CuI, it was 22% yield when 3aa and KOtBu were used in NMP under MW conditions for 60 min. Base on the observed experimental results and pioneering reports,15,18 we have described a plausible mechanistic pathway in Scheme 2. Oxidation addition of Fe(acac)3 by the amine and pyridine functional units resulting in an organoiron complex A. At this stage, trans-metalation by copper complex B provides an intermediate Fe-species C, which could then undergo reductive elimination to forge the new carbon–carbon triple bond, thus yielding the compound 3aa. After furnished the typical Sonogashira coupling, the desired 7-azaindole was generated under the microwave condition.
Conclusions
In summary, we have described the synthesis of biologically relevant 7-azaindole ring systems using an iron-catalyzed cyclic reaction under microwave irradiation. This approach provides a rapid and economical access to a diverse range of 7-azaindoles. The method employs readily available reagents and possesses broad scope and good tolerance of functional group, iron catalysis could be an interesting. Further efforts to utilize these compounds as versatile building blocks for assembling interesting heterocyclic molecules which can be applied in medicinal chemistry research are currently underway in our laboratories.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is supported by the National Natural Science Foundation of China (21867004), State Key Laboratory of Functions and Applications of Medicinal Plants (No. FAMP201707K) and (No. FAMP201801K).Notes and references
- (a) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed; (b) P. Appukkuttan, V. P. Mehta and E. V.-d. Eycken, Chem. Soc. Rev., 2010, 39, 1467 RSC; (c) M. Driowya, A. Saber, H. Marzaq, L. Demange, K. Bougrin and R. Benhida, Molecules, 2016, 21, 1032 CrossRef PubMed.
- Z. Yang, J. Wang, L. Peng, X. You and H. Cui, Tetrahedron Lett., 2016, 57, 5219 CrossRef CAS.
- D. Roy and Y. Uozumi, Adv. Synth. Catal., 2018, 360, 602 CrossRef CAS.
- M. B. Schuetz, L. Xiao, T. Lehnen, T. Fischer and S. Mathur, Int. Mater. Rev., 2018, 63, 341 CrossRef CAS.
- S. R. Walker, E. J. Carter, B. C. Huff and J. C. Morris, Chem. Rev., 2009, 109, 3080 CrossRef CAS PubMed.
- E. Spangenberg, P. L. Severson, L. A. Hohsfield, J. Crapser, J. Zhang, E. A. Burton, Y. Zhang, W. Spevak, J. Lin, N. Y. Phan, G. Habets, A. Rymar, G. Tsang, J. Walters, M. Nespi, P. Singh, S. Broome, P. Ibrahim, C. Zhang, G. Bollag, B. L. West and K. N. Green, Nat. Commun., 2019, 10, 3758 CrossRef PubMed.
- R. F. Goundry, K. Dai, M. Gonzalez, D. Legg, A. Kearney-McMullan, J. Morrison, A. Stark, P. Siedlecki, P. Tomlin and J. Yang, Org. Process Res. Dev., 2019, 23, 1333 CrossRef.
- N. D. Adams, J. L. Adams, J. L. Burgess, A. M. Chaudhari, R. A. Copeland, C. A. Donatelli, D. H. Drewry, K. E. Fisher, T. Hamajima, M. A. Hardwicke, W. F. Huffman, K. K. Koretke-Brown, Z. V. Lai, O. B. McDonald, H. Nakamura, K. A. Newlander, C. A. Oleykowski, C. A. Parrish, D. R. Patrick, R. Plant, M. A. Sarpong, K. Sasaki, S. J. Schmidt, D. J. Silva, D. Sutton, J. Tang, C. S. Thompson, P. J. Tummino, J. Wang, H. Xiang, J. Yang and D. Dhanak, J. Med. Chem., 2010, 53, 3973 CrossRef CAS PubMed.
- (a) S. Zhao and S. Wang, Chem. Soc. Rev., 2010, 39, 3142 RSC; (b) W. D. Tap, Z. A. Wainberg, S. P. Anthony, P. N. Ibrahim, C. Zhang, J. H. Healey, B. Chmielowski, A. P. Staddon, A. L. Cohn, G. I. Shapiro, V. L. Keedy, A. S. Singh, I. Puzanov, E. L. Kwak, A. J. Wagner, D. D. von-Hoff, G. J. Weiss, R. K. Ramanathan, J. Zhang, G. Habets, Y. Zhang, E. A. Burton, G. Visor, L. Sanftner, P. Severson, H. Nguyen, M. J. Kim, A. Marimuthu, G. Tsang, R. Shellooe, C. Gee, B. L. West, P. Hirth, K. Nolop, M. van-de-Rijn, H. H. Hsu, C. Peterfy, P. S. Lin, S. Tong-Starksen and G. Bollag, N. Engl. J. Med., 2015, 373, 428 CrossRef CAS PubMed; (c) T. Irie and M. Sawa, Chem. Pharm. Bull., 2018, 66, 29 CrossRef CAS PubMed; (d) W. Li and L. Dong, Adv. Synth. Catal., 2018, 360, 1104 CrossRef CAS; (e) N. Sharma and Anurag, Mini-Rev. Med. Chem., 2019, 19, 727 CrossRef CAS PubMed.
- (a) L. Yan, H. Wang, Y. Chen, Z. Li and Y. Pei, Mol. Diversity, 2018, 22, 323 CrossRef CAS PubMed; (b) L. Yan, M. Deng, A. Chen, Y. Li, W. Zhang, Z. Du, C. Dong, B. Meunier and H. Chen, Tetrahedron Lett., 2019, 60, 1359 CrossRef CAS.
- F. Popowycz, J. Y. Merour and B. Joseph, Tetrahedron, 2007, 63, 8689 CrossRef CAS.
- (a) T. Lessing, F. Sterzenbach and T. J. J. Mueller, Synlett, 2015, 26, 1217 CrossRef CAS; (b) S. I. Purificacao, M. J. D. Pires, R. Rippel, A. S. Santos and M. M. B. Marques, Org. Lett., 2017, 19, 5118 CrossRef CAS PubMed; (c) E. K. Yum and K. B. Hong, Tetrahedron, 2017, 73, 6581 CrossRef CAS.
- T. Cernak, K. D. Dykstra, S. Tyaqarajan, P. Vachal and S. W. Krska, Chem. Soc. Rev., 2016, 45, 546 RSC.
- A. Piontek, E. Bisz and M. Szostak, Angew. Chem., Int. Ed. Engl., 2018, 57, 11116 CrossRef CAS PubMed.
- J. Mao, G. Xie, M. Wu, J. Guo and S. Ji, Adv. Synth. Catal., 2008, 350, 2477 CrossRef CAS.
- A. Carpita, A. Ribecai and P. Stabile, Tetrahedron, 2010, 66, 7169 CrossRef CAS.
- T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed.
- (a) S. Handa, B. Jin, P. P. Bora, Y. Wang, X. Zhang, F. Gallou, J. Reilly and B. H. Lipshutz, ACS Catal., 2019, 9, 2423 CrossRef CAS; (b) K. S. Sindhu, A. P. Thankachan, A. M. Thomas and G. Anilkumar, ChemistrySelect, 2016, 1, 556 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08742g |
| This journal is © The Royal Society of Chemistry 2019 |