Theanine attenuates memory impairments induced by klotho gene depletion in mice
Bao Trong
Nguyen†
a,
Naveen
Sharma†
a,
Eun-Joo
Shin
*a,
Ji Hoon
Jeong
b,
Sung Hoon
Lee
c,
Choon-Gon
Jang
d,
Seung-Yeol
Nah
e,
Toshitaka
Nabeshima
fg,
Yukio
Yoneda
h and
Hyoung-Chun
Kim
 *a
*a
aNeuropsychopharmacology and Toxicology Program, College of Pharmacy, Kangwon National University, Chunchon 24341, Republic of Korea. E-mail: kimhc@kangwon.ac.kr; shinej@kangwon.ac.kr; Fax: +82 33 259 5631; Fax: +82 33 259 5631; Tel: +82 33 250 6917 Tel: +82 33 250 6922
bDepartment of Pharmacology, College of Medicine, Chung-Ang University, Seoul 06974, Republic of Korea
cDepartment of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 06974, Republic of Korea
dDepartment of Pharmacology, School of Pharmacy, Sungkyunkwan University, Suwon 16419, Korea
eGinsentology Research Laboratory and Department of Physiology, College of Veterinary Medicine and Bio/Molecular Informatics Center, Konkuk University, Seoul 05029, Republic of Korea
fAdvanced Diagnostic System Research Laboratory, Fujita Health University Graduate School of Health Sciences, Aichi 470-1192, Japan
gAino University, Ibaragi, 567-0012, Japan
hSection of Prophylactic Pharmacology, Kanazawa University Venture Business Laboratory, Kanazawa, Ishikawa 920-1192, Japan
First published on 14th December 2018
Abstract
Theanine (γ-glutamylethylamide), an amino acid in tea, is a putative neuroprotective and antioxidant compound capable of improving lifespan and cognitive function. Because we previously reported cognitive dysfunction in klotho mutant mice via down-regulation of janus kinase 2 (JAK2) and signal transducer and activator of transcription3 (STAT3), M1 muscarinic cholinergic receptor (M1 mAChR), and ERK signaling, we, therefore, investigated whether self-administration of theanine affects memory dysfunction in response to klotho gene depletion in mice, and whether theanine modulates the JAK2/STAT3, M1 mAChR, and ERK signaling network. Theanine significantly attenuated memory impairments in klotho mutant mice. Moreover, theanine self-administration significantly attenuated inhibitions of JAK2/STAT3 phosphorylation, M1 mAChR expression, and ERK1/2 phosphorylation in the hippocampus of klotho mutant mice. Consistently, AG490, a JAK2/STAT3 inhibitor, dicyclomine, an M1 mAChR antagonist, or U0126, an ERK1/2 inhibitor, significantly counteracted theanine-induced attenuation of memory impairment induced by klotho gene depletion in mice. Our study suggests that theanine attenuates memory impairments in a genetic aging model via up-regulation of JAK2/STAT3, M1 mAChR, and ERK signaling.
1. Introduction
Theanine (γ-glutamylethylamide), a major amino acid present in green tea, has recently been reported to possess antioxidant1–3 and neuroprotective properties.4–6L-Theanine has been considered as an active form of theanine, which is associated with memory enhancing effects.7 Consistently, L-theanine improves memory in elderly persons,8 and also suppresses accelerated aging, memory impairments, and behavioral depression in aged mice.9,10The Klotho-mutant mouse, a specific aging model, rapidly undergoes premature aging including growth retardation, hypogonadotropic hypogonadism, skin atrophy, sarcopenia, hearing disturbance, motor neuron degeneration, and memory impairments.11–13 Moreover, the klotho gene is downregulated with aging, whereas overexpression of the klotho gene significantly extends the lifespan of the organism.14 Thus, klotho mutant mice are considered to be a novel animal model for aging.13,15,16 We first demonstrated memory impairments in klotho mutant mice,15 suggesting that the klotho gene might be essential for maintaining memory function in aging organisms.15,16
In our previous study, we proposed that the inactivation of janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling axis and downregulation of M1 muscarinic acetylcholine receptor (mAChR) play a mechanistic role in memory impairments in klotho mutant mice.16 However, little is known about the effect of theanine on klotho gene depletion-induced memory dysfunction. Therefore, the present study was designed to investigate the effect of theanine against klotho gene depletion-induced memory dysfunction in mice. We observed here that theanine administration exerts memory enhancing activity in response to klotho gene depletion in mice via up-regulation of JAK2/STAT3 phosphorylation as well as M1 mAChR, finally resulting in enhanced extracellular signal-regulated kinase (ERK) phosphorylation.
2. Materials and methods
2.1. Animals
All animals were treated in accordance with the National Institutes of Health (NIH) Guide for the Humane Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1985; http://www.dels.nas.edu/ila). All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Kangwon National University and approved by the Animal Ethics Committee of Kangwon National University (approval No. KW-170222-3). Animal welfare was maximized to minimize the suffering as much as possible during experiments with live subjects. Mice were maintained under a 12 h light:12 h dark cycle and fed ad libitum. Since klotho mutant mice are infertile, wild-type and klotho mutant mice were generated by crossing heterozygous klotho mutant mice.15–17 The mice were screened by polymerase chain reaction (PCR) analysis using DNA extracted from tail specimens.Prior to weaning, tail specimens were collected from each animal, and DNA was extracted for genotype wild-type- and klotho mutant mice by polymerase chain reaction (PCR) using primer pairs specific for each genotype; 5′-TTGTGGAGATTGGAAGTGGACGAAAGAG-3′ (forward), 5′-CGCCCCGACCGGAGCTGAGAGTA-3′ (klotho mutant reverse), and 5′-CTGGACCCCCTGAAGCTGGAGTTAC-3′ (wild-type reverse).15,17 The products were amplified in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) using the following PCR parameters: an initial denaturation at 94 °C for 5 min, and then 30 cycles of 94 °C for 30 s, 65 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min, followed by electrophoresis on 1% agarose gels with ethidium bromide and photography under ultraviolet (UV) light. Six-week-old wild-type- or klotho mutant mice were used in the present study.
2.2. Drug treatment
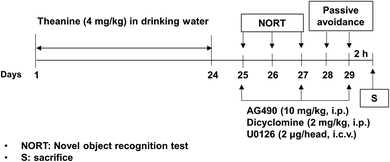
Theanine (Taiyo Kagaku Co., Ltd, Japan) was dissolved in drinking water, and dicyclomine hydrochloride (an M1 mAChR antagonist; Sigma-Aldrich) was dissolved in sterile saline, immediately before use. The JAK2/STAT3 inhibitor AG490 (Tocris Bioscience, Ellisville, MO, USA) or ERK1/2 inhibitor U0126 (Tocris Bioscience; Avonmouth, Bristol, UK; 1144) was dissolved in dimethyl sulfoxide (DMSO) respectively, in sterile saline immediately before use at a concentration of 2 mg ml−1.16,18 The final DMSO concentration was 10% (v/v).According to the experimental design shown in Fig. 1, mice (35–40 g per mouse) received theanine (4%) approximately about 4 mg kg−1 day−1, calculated by the water intake (4–4.5 ml per mouse), in drinking water for 24 consecutive days.1,19 Mice received AG490 (10 mg kg−1, i.p.), dicyclomine (2 mg kg−1, i.p.) 30 min before, or U0126 (2 μg per i.c.v. per head) 1 h before every memory test on day 25, 27, and 29. A neurochemical study (i.e., expressions of the cholinergic receptor and ERK1/2, JAK2/STAT3) from hippocampal tissues was conducted, just after the passive avoidance test (PAT). Mice were killed 2 h after the memory test for western blot analyses (p-JAK2, p-STAT3, mAChRs, nAChRs, and ERK1/2).
2.3. Guide cannula implantation and intracerebroventricular infusion
A stainless steel guide cannula (AG-4; Eicom, Kyoto, Japan) was implanted into the right lateral ventricle (stereotaxic coordinates: 0.5 mm posterior to bregma, 1 mm right of the midline, and 2 mm ventral to the dura) as described before.20,21 Microinfusion into the lateral ventricle was performed through a microinfusion cannula (AMI-4, Eicom) at a rate of 1 μL min−1 using a microinjection pump (CMA/100, CMA, Solna, Sweden). The microinfusion cannula was kept in place for 1 min after infusion to avoid backflow.2.4. Novel object recognition test (NORT)
NORT was performed as previously described.16,17,22 The mice were allowed to individually habituate to an open box (40 × 40 × 40 cm) for 10 min a day before the training session. During the training session, two novel objects were placed in the open box, and the mice were allowed to explore for 10 min under moderate light (10 lx). The exploration time for each object was recorded. A test trial was conducted 24 h after the training session. During the retention session, the animals were placed in the same box as they had been placed in for the training session, and one of the familiar objects used during training was replaced with a novel object. The mice were then allowed to explore freely for 10 min. The preference index during the retention session and the ratio of the amount of time spent exploring the novel object to the total time spent exploring both objects were used to measure memory function in the mice.2.5. Passive avoidance test
Passive avoidance was measured by using a Gemini Avoidance System (San Diego Instrument, San Diego, CA), which consists of two-compartment shuttle chambers with a constant current shock generator. The step-through passive avoidance apparatus consisted of one clear and one dark chamber separated by a guillotine door. The floors of both the clear (12 × 10 × 12 cm) and dark chambers (12 × 10 × 12 cm) were made of 2 mm stainless steel rods spaced 0.5 cm apart. A 50 W lamp positioned 1 m above both chambers illuminated the apparatus. On an acquisition trial, each mouse was placed into the start chamber, which remained dark. After 20 s, the chamber light was illuminated and the door was opened for the mouse to move into the dark chamber freely. Immediately after the mouse entered the dark chamber, the door was closed, and an inescapable scrambled electric shock (0.8 mA, 2 s, once) was delivered through the floor grid. Then, the mouse was returned to its home cage. Twenty-four hours later, each mouse was placed in the start chamber again (retention trial). The interval between placement in the lighted chamber and entry into the dark chamber was measured as the step-through latency in both acquisition and retention trials (maximum 300 s).17,232.6. Western blot analysis
Western blot was performed as described previously.16–18,22 Tissues were homogenized in lysis buffer, containing 200 mM Tris HCl (pH 6.8), 1% SDS, 5 mM ethylene glycol tetraacetic acid, 5 mM ethylenediaminetetraacetic acid, 10% glycerol, 1 × phosphatase inhibitor cocktail I, and 1 × protease inhibitor cocktail. The supernatant fraction was subsequently centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min. Proteins (20–50 μg per lane) were separated by 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Following transfer, membranes were preincubated with 5% non-fat milk and incubated overnight at 4 °C with the primary antibody against β-actin (1
000g for 30 min. Proteins (20–50 μg per lane) were separated by 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Following transfer, membranes were preincubated with 5% non-fat milk and incubated overnight at 4 °C with the primary antibody against β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Sigma-Aldrich), anti-JAK2 (1
000; Sigma-Aldrich), anti-JAK2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-phospho-JAK2 (1
500, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-phospho-JAK2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Cell Signaling Technology, Inc.), anti-STAT3 (1
500, Cell Signaling Technology, Inc.), anti-STAT3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Cell Signaling Technology, Inc.), anti-phospho-STAT3 (1
500, Cell Signaling Technology, Inc.), anti-phospho-STAT3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Cell Signaling Technology, Inc.), anti-muscarinic M1 receptor (1
1000, Cell Signaling Technology, Inc.), anti-muscarinic M1 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, abcam, Cambridge, UK), anti-muscarinic M2 receptor (1
1000, abcam, Cambridge, UK), anti-muscarinic M2 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-muscarinic M3 receptor mAChR (1
500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-muscarinic M3 receptor mAChR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Santa Cruz Biotechnology, Inc.), anti-muscarinic M4 receptor (1
500, Santa Cruz Biotechnology, Inc.), anti-muscarinic M4 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Chemicon International, Inc. Temecula, CA, USA), anti-muscarinic M5 receptor (1
1000, Chemicon International, Inc. Temecula, CA, USA), anti-muscarinic M5 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Santa Cruz Biotechnology, Inc.), anti-nicotinic α4 receptor (1
500, Santa Cruz Biotechnology, Inc.), anti-nicotinic α4 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Santa Cruz Biotechnology, Inc.), anti-nicotinic β2 receptor (1
500, Santa Cruz Biotechnology, Inc.), anti-nicotinic β2 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Santa Cruz Biotechnology, Inc.), anti-nicotinic α7 receptor (1
500, Santa Cruz Biotechnology, Inc.), anti-nicotinic α7 receptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, Santa Cruz Biotechnology, Inc.), anti-ERK (1
500, Santa Cruz Biotechnology, Inc.), anti-ERK (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, Cell Signaling Technology, Inc.), and anti-phospho-ERK (1
5000, Cell Signaling Technology, Inc.), and anti-phospho-ERK (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Cell Signaling Technology, Inc.). After incubation with the primary antibody, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit IgG (1
1000, Cell Signaling Technology, Inc.). After incubation with the primary antibody, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; GE Healthcare, Piscataway, NJ, USA), anti-mouse IgG (1
1000; GE Healthcare, Piscataway, NJ, USA), anti-mouse IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; Sigma, St Louis, MO, USA), or anti-goat IgG (1
1000; Sigma, St Louis, MO, USA), or anti-goat IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; Sigma) for 2 h. Subsequent visualization was performed using the ECL plus® enhanced chemiluminescence system (GE Healthcare). Relative band intensities were quantified using PhotoCapt MW (version 10.01 for Windows; Vilber Lourmat, Marne la Vallée, France), and each intensity was then normalized to β-actin intensity.
1000; Sigma) for 2 h. Subsequent visualization was performed using the ECL plus® enhanced chemiluminescence system (GE Healthcare). Relative band intensities were quantified using PhotoCapt MW (version 10.01 for Windows; Vilber Lourmat, Marne la Vallée, France), and each intensity was then normalized to β-actin intensity.
2.7. Statistics
Data were analyzed using IBM SPSS version 21.0 (IBM). Three-way [gene × treatment × inhibitor (treatment)] analyses of variance (ANOVA) were performed for the statistical analysis followed by post hoc Fisher's least significant difference pairwise comparisons tests. A value of p < 0.05 was taken to indicate statistical significance.3. Results
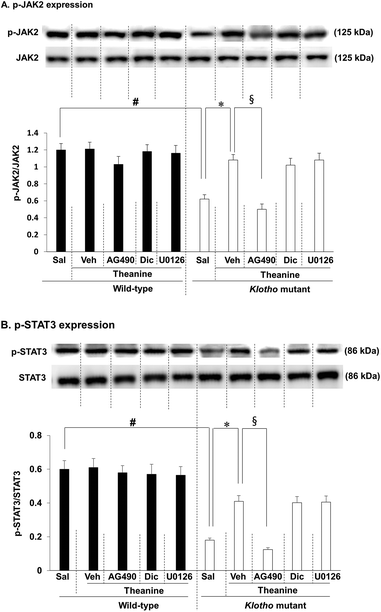
3.1. Effects of theanine on phospho-JAK2 (p-JAK2) and phospho-STAT3 (p-STAT3) expression in the hippocampus of klotho mutant mice
As we previously reported, JAK2/STAT3 signaling is initially down-regulated in klotho mutant mice;16 here we investigated whether theanine affects klotho gene depletion-induced decrease in p-JAK2/p-STAT3 expression. As shown in the experimental design in Fig. 1, we first examined p-JAK2 (Fig. 2A) and p-STAT3 (Fig. 2B) expression. p-JAK2/p-STAT3 were significantly decreased (p-JAK2, p < 0.01; p-STAT3, p < 0.01) in klotho mutant mice. In contrast, theanine treatment significantly attenuated klotho mutant-induced decrease in p-JAK2 (Fig. 2A; p < 0.01) and p-STAT3 (Fig. 2B; p < 0.01) expressions. This pharmacological activity of theanine was significantly counteracted (p < 0.01) by AG490, a JAK2/STAT3 inhibitor, whereas dicyclomine, an M1 mAChR antagonist, or U0126, an ERK inhibitor, did not affect theanine mediated potential, indicating that JAK2/STAT3 is upstream of M1 mAChR and ERK signaling.3.2. Effects of theanine on cholinergic receptor expression in the hippocampus of klotho mutant mice
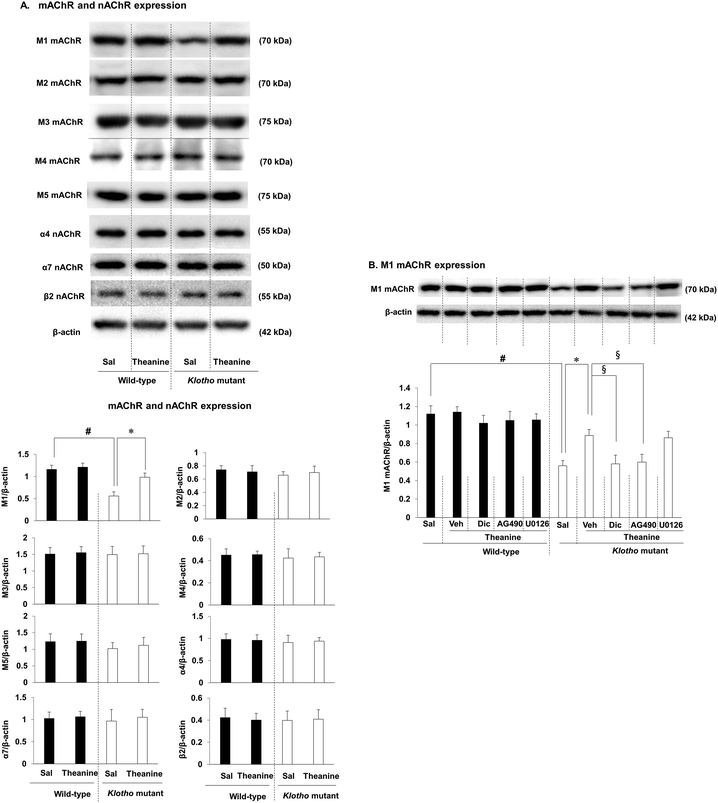
We next examined whether theanine affects muscarinic acetylcholine receptor (mAChR) and nicotinic acetylcholine receptor (nAChR) expressions in klotho mutant mice. As we demonstrated,16 M1 mAChR out of M1–M5 mAChRs was selectively and significantly decreased (p < 0.01) in klotho mutant mice, whereas there was no significant change in nAChR subtypes (α4 nAChR, α7 nAChR, β2 nAChR, and α4β2 nAChR) (Fig. 3A). Theanine treatment significantly and selectively attenuated (p < 0.01) the decrease in M1 mAChR expression in klotho mutant mice (Fig. 3B). The effect of theanine was consistently counteracted (p < 0.01) by AG490, a JAK2/STAT3 inhibitor, or dicyclomine, an M1 mAChR antagonist, whereas U0126, an ERK inhibitor, did not significantly alter theanine-mediated M1 mAChR expression.3.3. Effect of theanine on phospho-ERK (p-ERK) expression in the hippocampus of klotho mutant mice
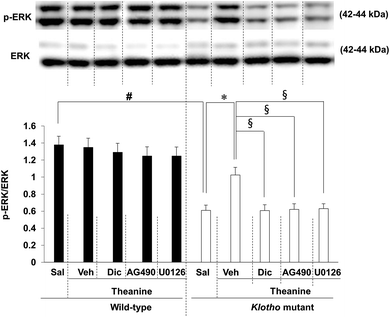
Previously we demonstrated that klotho gene depletion-induced memory impairments require inactivation of ERK1/2 signaling.16,17 Consistently, theanine treatment significantly attenuated (p < 0.01) klotho gene depletion-mediated reduction in p-ERK1/2 levels (Fig. 4). Theanine-mediated attenuation was significantly counteracted (p < 0.01) by AG490, a JAK2/STAT3 inhibitor, dicyclomine, an M1 mAChR antagonist, or U0126, an ERK inhibitor in klotho mutant mice.3.4. Effect of theanine on memory impairments in klotho mutant mice
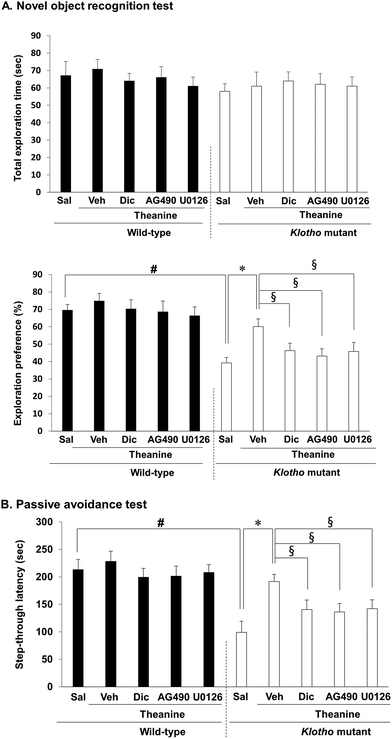
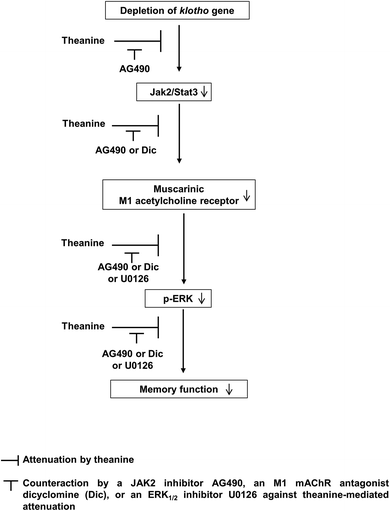
We demonstrated that genetic depletion of the klotho gene produces memory impairments in mice.16,17 Therefore, we employed the novel object recognition test (NORT) and passive avoidance test (PAT) to investigate whether theanine modulates memory function in klotho mutant mice. The total exploration time was not altered among all groups. Klotho gene depletion significantly decreased novel object recognition memory (p < 0.01, Fig. 5A), and passive avoidance performance (p < 0.01, Fig. 5B) in mice. Theanine treatment significantly improved klotho gene depletion-induced memory impairments (NORT, p < 0.01; PAT, p < 0.01).Importantly, theanine-mediated memory enhancing potential was significantly counteracted (p < 0.05) by AG490, a JAK2/STAT3 inhibitor, dicyclomine, an M1 mAChR antagonist, or U0126, an ERK inhibitor (Fig. 5A and B), suggesting that theanine-mediated memory enhancing activity requires up-regulation of JAK2-STAT3/M1 mAChR, and ERK1/2 signaling in response to klotho gene depletion in mice (Fig. 6).
4. Discussion
In our previous study, we proposed that klotho gene deficiency impairs memory function via inactivation of JAK2/STAT3 that might be associated with cholinergic dysfunction and inactivation of ERK signaling.16 In the present study, we observed that theanine treatment improves memory function in klotho gene depletion in mice. We found here that theanine treatment activates JAK2/STAT3 phosphorylation. Theanine also selectively potentiates M1 mAChR and ERK signaling against memory impairments in klotho mutant mice. Importantly, the pharmacological potential mediated by theanine was inhibited by AG490, a JAK2/STAT3 inhibitor, dicyclomine, an M1 mAChR antagonist, or U0126, an ERK inhibitor, suggesting that the JAK2/STAT3, M1 mAChR, and ERK signaling pathway is crucial for the memory enhancing activity of theanine against klotho gene depletion in mice.Increasing evidence suggested that activation of the JAK2/STAT3 pathway is essential for improving spatial learning and memory.24 Chiba et al. (2009) proposed that pharmacological inhibition of JAK2/STAT3 signaling induces spatial working memory loss by downregulating choline acetyltransferase and M1 mAChR.25 Additionally, the hippocampal p-STAT3 immunoreactivity of young subjects is higher than that of older, healthy humans and rodents.25 Similarly, we also observed that mountain cultivated ginseng protects against memory impairments induced by the neurotoxin trimethyltin in mice via activation of JAK2/STAT3/ERK signaling.18 In line with our previous studies,16,18 it is plausible that activation of JAK2/STAT3 is important for theanine-mediated memory enhancing activity.
mAChRs are G-protein-coupled receptors, which are subdivided into 5 subtypes (i.e., M1–M5) based on their coupling to the signal transduction pathway.26 mAChRs are involved in neuronal plasticity,27 and are associated with memory function.22,28 In particular, impairment in M1 mAChR mediated signaling leads to memory impairments in klotho mutant mice.16 Moreover, it has been reported that M1 mAChR activation improves memory function via JAK2/STAT3 signaling.16,28 In addition, M1 mAChR-mediated activation of ERK1/2 is critical for memory function.16,22,28 Therefore, we propose that theanine improves memory function via up-regulation of M1 mAChR through the JAK2/STAT3 pathway in klotho mutant mice.
The ERK1/2 signaling pathway is well known to exhibit multiple functions, which include cell survival and mammalian-associative learning.29,30 M1 mAChR significantly activates ERK1/2 signaling in the hippocampus,17,22,31 which might improve memory function in rodents.17,28 In addition, activation of ERK1/2 in the prefrontal cortex of mice after exposure to a novel object is crucial for long-term recognition memory,32–34 suggesting that down-regulation of ERK1/2 is critical for memory dysfunction,33,34 whereas up-regulation of ERK1/2 improves memory function.17,18,22,28 Taken together, our results suggest that activation of ERK1/2 signaling via up-regulation of M1 mAChR contributes to theanine-mediated memory improvement in response to klotho gene depletion in mice.
5. Conclusion
We demonstrated here that theanine protects against memory dysfunction induced by klotho gene deficiency. The results showed that theanine significantly attenuates down-regulations in p-JAK2/p-STAT3, M1 mAChR, and ERK1/2 signaling in klotho mutant mice. Finally, we are the first to propose that up-regulation of p-JAK2/p-STAT3, M1 mAChR, and ERK1/2 signaling is important for the memory enhancing activity of theanine in response to klotho gene depletion in mice, a genetic animal model of aging.Conflicts of interest
All the authors declare that there is no conflict of interest.Acknowledgements
This study was supported by the Basic Science Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, & Future Planning (#NRF-2013R1A1A2060894 and #NRF-2016R1A1A1A050055201), Republic of Korea. Bao Trong Nguyen and Naveen Sharma were supported by the BK21 PLUS program. We would like to express our thanks to Taiyo Kagaku Co., Ltd for the generous donation of theanine.References
- T. I. Kim, Y. K. Lee, S. G. Park, I. S. Choi, J. O. Ban, H. K. Park, S. Y. Nam, Y. W. Yun, S. B. Han, K. W. Oh and J. T. Hong, L-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways, Free Radicals Biol. Med., 2009, 47, 1601–1610 CrossRef CAS
.
- H. S. Cho, S. Kim, S. Y. Lee, J. A. Park, S. J. Kim and H. S. Chun, Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death, NeuroToxicology, 2008, 29, 656–662 CrossRef CAS PubMed
.
- T. Yokozawa and E. Dong, Influence of green tea and its three major components upon low-density lipoprotein oxidation, Exp. Toxicol. Pathol., 1997, 49, 329–335 CrossRef CAS
.
- T. Takarada, N. Nakamichi, T. Kakuda, R. Nakazato, H. Kokubo, S. Ikeno, S. Nakamura, E. Hinoi and Y. Yoneda, Daily oral intake of theanine prevents the decline of 5-bromo-2′-deoxyuridine incorporation in hippocampal dentate gyrus with concomitant alleviation of behavioral abnormalities in adult mice with severe traumatic stress, J. Pharmacol. Sci., 2015, 127, 292–297 CrossRef CAS
.
- K. Rezai-Zadeh, G. W. Arendash, H. Hou, F. Fernandez, M. Jensen, M. Runfeldt, R. D. Shytle and J. Tan, Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice, Brain Res., 2008, 1214, 177–187 CrossRef CAS PubMed
.
- J. T. Hong, S. R. Ryu, H. J. Kim, J. K. Lee, S. H. Lee, D. B. Kim, Y. P. Yun, J. H. Ryu, B. M. Lee and P. Y. Kim, Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury, Brain Res. Bull., 2000, 53, 743–749 CrossRef CAS
.
- N. Egashira, N. Ishigami, F. Pu, K. Mishima, K. Iwasaki, K. Orito, R. Oishi and M. Fujiwara, Theanine prevents memory impairment induced by repeated cerebral ischemia in rats, Phytother. Res., 2008, 22, 65–68 CrossRef CAS
.
- T. Kakuda, Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction, Pharmacol. Res., 2011, 64, 162–168 CrossRef CAS PubMed
.
- Y. Zhao and B. Zhao, Natural antioxidants in prevention and management of Alzheimer's disease, Front. Biosci., Elite Ed., 2012, 4, 794–808 CrossRef
.
- K. Unno, K. Fujitani, N. Takamori, F. Takabayashi, K. Maeda, H. Miyazaki, N. Tanida, K. Iguchi, K. Shimoi and M. Hoshino, Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice, Free Radical Res., 2011, 45, 966–974 CrossRef CAS PubMed
.
- S. J. Park, Y. H. Chung, J. H. Lee, D. K. Dang, Y. Nam, J. H. Jeong, Y. S. Kim, T. Nabeshima, E. J. Shin and H. C. Kim, Growth Hormone-Releaser Diet Attenuates Cognitive Dysfunction in Klotho Mutant Mice via Insulin-Like Growth Factor-1 Receptor Activation in a Genetic Aging Model, Endocrinol. Metab., 2014, 29, 336–348 CrossRef PubMed
.
- M. Kuro-o, Klotho, Pflugers Arch., 2010, 459, 333–343 CrossRef CAS PubMed
.
- M. Kuro-o, Y. Matsumura, H. Aizawa, H. Kawaguchi, T. Suga, T. Utsugi, Y. Ohyama, M. Kurabayashi, T. Kaname, E. Kume, H. Iwasaki, A. Iida, T. Shiraki-Iida, S. Nishikawa, R. Nagai and Y. I. Nabeshima, Mutation of the mouse klotho gene leads to a syndrome resembling ageing, Nature, 1997, 390, 45–51 CrossRef CAS PubMed
.
- H. Kurosu, M. Yamamoto, J. D. Clark, J. V. Pastor, A. Nandi, P. Gurnani, O. P. McGuinness, H. Chikuda, M. Yamaguchi, H. Kawaguchi, I. Shimomura, Y. Takayama, J. Herz, C. R. Kahn, K. P. Rosenblatt and M. Kuro-o, Suppression of aging in mice by the hormone Klotho, Science, 2005, 309, 1829–1833 CrossRef CAS PubMed
.
- T. Nagai, K. Yamada, H. C. Kim, Y. S. Kim, Y. Noda, A. Imura, Y. Nabeshima and T. Nabeshima, Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress, FASEB J., 2003, 17, 50–52 CrossRef CAS
.
- S. J. Park, E. J. Shin, S. S. Min, J. An, Z. Li, Y. Hee Chung, J. Hoon Jeong, J. H. Bach, S. Y. Nah, W. K. Kim, C. G. Jang, Y. S. Kim, Y. Nabeshima, T. Nabeshima and H. C. Kim, Inactivation of JAK2/STAT3 signaling axis and downregulation of M1 mAChR cause cognitive impairment in klotho mutant mice, a genetic model of aging, Neuropsychopharmacology, 2013, 38, 1426–1437 CrossRef CAS
.
- E. J. Shin, Y. H. Chung, H. L. Le, J. H. Jeong, D. K. Dang, Y. Nam, M. B. Wie, S. Y. Nah, Y. Nabeshima, T. Nabeshima and H. C. Kim, Melatonin attenuates memory impairment induced by Klotho gene deficiency via interactive signaling between MT2 receptor, ERK, and Nrf2-related antioxidant potential, Int. J. Neuropsychopharmacol., 2014, 18 DOI:10.1093/ijnp/pyu105
.
- T. T. Tu, N. Sharma, E. J. Shin, H. Q. Tran, Y. J. Lee, S. Y. Nah, H. P. Tran, J. H. Jeong, S. K. Ko, J. K. Byun and H. C. Kim, Treatment with Mountain-Cultivated Ginseng Alleviates Trimethyltin-Induced Cognitive Impairments in Mice via IL-6-Dependent JAK2/STAT3/ERK Signaling, Planta Med., 2017, 83, 1342–1350 CrossRef CAS PubMed
.
- X. Tian, L. Sun, L. Gou, X. Ling, Y. Feng, L. Wang, X. Yin and Y. Liu, Protective effect of l-theanine on chronic restraint stress-induced cognitive impairments in mice, Brain Res., 2013, 1503, 24–32 CrossRef CAS PubMed
.
- T. T. Tu, N. Sharma, E. J. Shin, H. Q. Tran, Y. J. Lee, J. H. Jeong, S. Y. Nah, H. P. Tran, J. K. Byun, S. K. Ko and H. C. Kim, Ginsenoside Re protects trimethyltin-induced neurotoxicity via activation of IL-6-mediated phosphoinositol 3-kinase/Akt signaling in mice, Neurochem. Res., 2017, 42, 3125–3139 CrossRef CAS PubMed
.
- E. J. Shin, S. W. Shin, T. T. Nguyen, D. H. Park, M. B. Wie, C. G. Jang, S. Y. Nah, B. W. Yang, S. K. Ko, T. Nabeshima and H. C. Kim, Ginsenoside Re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase Cdelta gene, Mol. Neurobiol., 2014, 49, 1400–1421 CrossRef CAS PubMed
.
- H. N. Mai, N. Sharma, E. J. Shin, B. T. Nguyen, P. T. Nguyen, J. H. Jeong, C. G. Jang, E. H. Cho, S. Y. Nah, N. H. Kim, T. Nabeshima and H. C. Kim, Exposure to far-infrared rays attenuates methamphetamine-induced recognition memory impairment via modulation of the muscarinic M1 receptor, Nrf2, and PKC, Neurochem. Int., 2018, 116, 63–76 CrossRef CAS PubMed
.
- C. H. Jin, E. J. Shin, J. B. Park, C. G. Jang, Z. Li, M. S. Kim, K. H. Koo, H. J. Yoon, S. J. Park, W. C. Choi, K. Yamada, T. Nabeshima and H. C. Kim, Fustin flavonoid attenuates beta-amyloid (1–42)-induced learning impairment, J. Neurosci. Res., 2009, 87, 3658–3670 CrossRef CAS PubMed
.
- Z. H. Zhang, L. J. Yu, X. C. Hui, Z. Z. Wu, K. L. Yin, H. Yang and Y. Xu, Hydroxy-safflor yellow A attenuates Abeta(1)(-)(4)(2)-induced inflammation by modulating the JAK2/STAT3/NF-kappaB pathway, Brain Res., 2014, 1563, 72–80 CrossRef CAS PubMed
.
- T. Chiba, M. Yamada, J. Sasabe, K. Terashita, M. Shimoda, M. Matsuoka and S. Aiso, Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons, Mol. Psychiatry, 2009, 14, 206–222 CrossRef CAS PubMed
.
- P. J. Nathan, J. Watson, J. Lund, C. H. Davies, G. Peters, C. M. Dodds, B. Swirski, P. Lawrence, G. D. Bentley, B. V. O'Neill, J. Robertson, S. Watson, G. A. Jones, P. Maruff, R. J. Croft, M. Laruelle and E. T. Bullmore, The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction, Int. J. Neuropsychopharmacol., 2013, 16, 721–731 CrossRef CAS PubMed
.
- D. Jerusalinsky, E. Kornisiuk and I. Izquierdo, Cholinergic neurotransmission and synaptic plasticity concerning memory processing, Neurochem. Res., 1997, 22, 507–515 CrossRef CAS
.
- B. K. Kim, H. Y. Tran, E. J. Shin, C. Lee, Y. H. Chung, J. H. Jeong, J. H. Bach, W. K. Kim, D. H. Park, K. Saito, T. Nabeshima and H. C. Kim, IL-6 attenuates trimethyltin-induced cognitive dysfunction via activation of JAK2/STAT3, M1 mAChR and ERK signaling network, Cell. Signalling, 2013, 25, 1348–1360 CrossRef CAS PubMed
.
- J. D. English and J. D. Sweatt, Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation, J. Biol. Chem., 1996, 271, 24329–24332 CrossRef CAS PubMed
.
- G. Pearson, F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman and M. H. Cobb, Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions, Endocr. Rev., 2001, 22, 153–183 CAS
.
- J. L. Berkeley, J. Gomeza, J. Wess, S. E. Hamilton, N. M. Nathanson and A. I. Levey, M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices, Mol. Cell. Neurosci., 2001, 18, 512–524 CrossRef CAS PubMed
.
- S. Blum, A. N. Moore, F. Adams and P. K. Dash, A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory, J. Neurosci., 1999, 19, 3535–3544 CrossRef CAS
.
- H. Kamei, T. Nagai, H. Nakano, Y. Togan, M. Takayanagi, K. Takahashi, K. Kobayashi, S. Yoshida, K. Maeda, K. Takuma, T. Nabeshima and K. Yamada, Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice, Biol. Psychiatry, 2006, 59, 75–84 CrossRef CAS PubMed
.
- T. V. Tran, E. J. Shin, L. T. T. Nguyen, Y. Lee, D. J. Kim, J. H. Jeong, C. G. Jang, S. Y. Nah, K. Toriumi, T. Nabeshima, K. Yamada and H. C. Kim, Protein kinase Cdelta gene depletion protects against methamphetamine-induced impairments in recognition memory and ERK1/2 signaling via upregulation of glutathione peroxidase-1 gene, Mol. Neurobiol., 2017 DOI:10.1007/s12035-017-0638-8
.
Footnote |
| † The first two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |