A combination of monoacylglycerol crystalline network and hydrophilic antioxidants synergistically enhances the oxidative stability of gelled algae oil
Qiang
Wang
 a,
Eric Andrew
Decker
b,
Jiajia
Rao
a,
Eric Andrew
Decker
b,
Jiajia
Rao
 c and
Bingcan
Chen
c and
Bingcan
Chen
 *c
*c
aDepartment of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China
bDepartment of Food Science, University of Massachusetts, Amherst, MA 01003, USA
cDepartment of Plant Sciences, North Dakota State University, Fargo, ND 58108, USA. E-mail: bingcan.chen@ndsu.edu; Tel: +(701) 231-9450
First published on 10th December 2018
Abstract
In this study, base algae oil was gelled through the formation of a crystal network using food-grade monoacylglycerol (MAG). The impact of the MAG concentration (5, 10, 20 wt%) and water content (0, 5 wt%) on the physical properties and oxidative stability of the gelled algae oil was systematically investigated. The antioxidative activity of 300 μM hydrophilic antioxidant, i.e., ascorbic acid and green tea extract, on the oxidative stability of the gelled algae oil by 20 wt% of MAG was also examined. The results obtained clearly showed that the melting temperature, melting of entropy, and complex modulus of the algae oil increased with increasing the MAG concentration. The addition of 5 wt% water could negatively affect the strength of the MAG crystal network, while a physically stable gel system could only be formed with 20 wt% MAG. The stronger crystal network formed by 20 wt% MAG retarded the lipid oxidation of algae oil due to the creation of a physical barrier to restrain the attack from oxygen. The addition of green tea extract could further synergize with the MAG crystalline network by forming a thermodynamic barrier to effectively quench the radicals, thus prolonging the oxidative stability of algae oil 4-fold longer than that of the base algae oil.
1. Introduction
Physically solidifying liquid oil into a semi-solid state using a gelator (i.e., edible materials with gelling capacity) instead of chemical modification, such as hydrogenation and interesterification, is a nontraditional structuring technique and has become an active area of research over the past decade.1,2 The fundamentals of transforming liquid oil into a self-standing and thermo-reversible solid oil by gelling process involves the aggregation of a gelator through its self-assembly and/or crystallization. The gelled oil formed by a gelator with a lower concentration (<10 wt%) is loosely defined as an oleogel since a greater concentration of gelator was also used.3 The development of a solid-like oil using a gelator has been gaining popularity in the food and pharmaceutical industry since it provides the formation of a plastic gel-like oil, which may substitute partially hydrogenated oil and other saturated fats.4 However, studies on the oxidative stability of gelled oil are scarce. To date, only a limited number of studies have been conducted to investigate the formation of gelled fish oil with an aim to improve its oxidative stability.5–7 These studies concluded that the solid-like system may restrain oxygen migration and thus enhance the fish oil oxidative stability.As a matter of fact, harsh thermal treatment is always associated with the oil gelling processing in light of the high melting point of the gelator. Only when the environmental temperature is higher than the glass transition temperature of the gelator can the liquid oil be entrapped in the network formed upon the crystalline of the melted gelator. Such a procedure could be a potential issue to attempts to deteriorate the chemical stability of oils in the solid state. Recent research has found that high temperature treatment adversely influences the oxidative stability of liquid oil entrapped in oleogel.3,8 For instance, canola oil oleogel formed by 15 wt% ethylcellulose went rancid extremely quickly, and with peroxides, the value was raised 5-fold after 10 min heating at 140 °C.3 Another study also indicated peroxides value of canola oil oleogel formed by 10 wt% waxes increased dramatically within 3 days storage under the accelerated conditions.9 Unlike the extremely high oxidative stability of high-melting point crystalline lipid hardstock produced by chemical modification, the observations derived from these studies indicated that the gelling process itself may accelerate the oxidation of gelled oil. This could be even worse when oxidation-prone PUFA predominates in the gelled oils. Thus, it is imperative to develop antioxidant strategies suitable for oil gelling preparation so that the gelled oil will possess the desired physical properties and oxidative stability.
Among most of the gelators, relatively low melting point monoacylglycerol (MAG) is the one that has been most widely used to structure liquid oil into the semi-solid state.10,11 However, most of the studies performed to date have primarily focused on the physical aspects of the MAG–oil binary system. Our previous study showed that the addition of MAG had no impact on stripped soybean oil oxidation in the absence of α-tocopherol; however, it could deteriorate the oil oxidative stability by suppressing the effectiveness of the lipophilic antioxidant α-tocopherol added.12 However, the role of MAG and its crystalline network on the oxidative stability of the gelled oil, especially an oil without antioxidants, has yet to be investigated. On the other hand, we also found the incorporation of natural hydrophilic antioxidants, including green tea extract (GET) and ascorbic acid (AA), exerted a superior protective efficiency more than rosmarinic acid, grape seed extract, and grape seed extract polymer against the oxidation of a water-in-base algae oil emulsion containing 5 wt% water.13 Green tea extract has also been reported to protect sunflower oil and its oil-in-water emulsions against oxidation.14 Since harsh thermal processing is a big concern for the oxidative stability of the gelled oil, we hereby present the role of the MAG crystalline network and the hydrophilic antioxidants on the oxidative stability of gelled base algae oil. The impact of water (5 wt%) on the efficacy of hydrophilic antioxidants on gelled algae oil was also investigated based on the assumption that a small amount of water can better dissolve hydrophilic antioxidants.
2. Materials and methods
2.1. Materials
Both base algae oil and green tea extract (>90% epigallocatechin gallate, Teavigo®) were obtained from DSM Nutritional Products Ltd (Columbia, MD, USA). According to the data supplied by the manufacturer, base algae oil is the refined oil without an added antioxidant and it contains >40% docosahexaenoic acid (DHA). Distilled monoacylglycerol (Dimodan® HP K-A, Lot#1140693449) made from edible, fully hydrogenated palm oil was a gift from Danisco (Danisco USA Inc.). Ascorbic acid and propanal were purchased from Sigma-Aldrich Co. HPLC grade methanol, n-hexane, and other chemicals were purchased from Fisher Scientific (Pittsburgh, PA, USA).2.2. Preparation of the gelled algae oil
The gelled algae oil was prepared by first adding different amounts of MAG (5, 10, and 20 wt%) into glass beakers containing algae oil. The beakers were then placed into a water bath with the temperature set at 75 °C, which was above the monoacylglycerol glass transition temperature (∼71 °C), in order to ensure the complete melt of the mixture; 5 wt% water and/or 300 μM hydrophilic antioxidant (i.e., ascorbic acid, green tea extract) were added into the melted mixture as needed. The melted mixture were then agitated using a glass rod gently before pouring into storage containers. For the physical tests, the melted liquid mixture was poured into disposable borosilicate glass tubes (16 mm × 125 mm) and cooled at room temperature for 20 min before refrigerating at 4 °C. After 7 days storage, the samples were prepared for testing of their physical properties. For the oxidative study, 1.00 mL of liquid mixture at 75 °C was pipetted into 10 mL GC vial which was then sealed by screw thread caps with inserted polytetrafluoroethylene (PTFE)/butyl rubber septa. The sample vials were cooled at room temperature for 20 min before refrigerating at 4 °C. After 24 h storage, all the capped GC vials were stored in a close box and stored in a 45 °C incubator room in the dark. In case the hydrophilic antioxidants were involved, 300 μM of ascorbic acid (AA) or green tea extract (GTE) was mixed with algae oil initially before adding MAG.2.3. Thermal behavior of gelled algae oil
Different scanning calorimetry (DSC) was used to identify any thermal transitions in the samples during heating. An aliquot of the sample (8–10 mg) was placed in an aluminum pan and hermetically sealed. An empty pan was used as a reference. The sample and reference pans were placed into the measurement block of the DSC (Q 1000, TA Instruments, New Castle, DE, USA) at room temperature, equilibrated at 0 °C for 2 min, and then heated to 90 °C at 2 °C min−1. This temperature range was selected to go from below to above the melting point of MAG.2.4. Rheological behaviors of gelled algae oil
The rheological behavior of gelled algae oil was determined using a Kinexus rotational rheometer (Malvern Instruments, Malvern, USA) at 25 °C. A concentric cylinder (code number: C25) measurement system was used, comprising a rotating inner cylinder, diameter 25 mm; static outer cylinder, diameter 27.5 mm. The samples were loaded into the rheometer measurement cell and allowed to equilibrate to 25 °C for 5 min. The viscoelastic behavior of the samples was recorded using a cone and plate geometry (the cone had a 4 cm diameter and 3.59° angle). A pseudo-linear viscoelastic region (LVR) was determined by oscillatory stress sweep, where G′ and G′′ were independent on the stress applied. The frequency sweep experiments were measured between 0.1 and 100 Hz and the complex modulus (G*) was determined. A temperature variation of 3° min−1 was imposed when characterizing the melting and crystallinity of the oleogels upon heating and cooling.2.5. Morphology of gelled algae oil
A polarized optical microscope (Nikon Eclipse E400, Nikon Corp., Japan) equipped with a heating stage (Linkham PE 94; Linkam Scientific Instruments, Tadworth, Surrey, U.K.) attached to a filter pump (Eheim Professional III, Deizisau, Germany) was used to record the microstructures of the gelled algae oil held at different temperatures during heating. The gelled algae oil samples were thoroughly smeared on a microscope slide by a glass rod, and covered by a cover slip. The sample slide was then loaded on the heating stage and heated from 20 °C to the desired temperature at a controlled heating rate of 5 °C min−1. Images were acquired at specific temperatures (25, 50, 55, 60, and 65 °C) using microscopy with a 40× objective lens (NA 0.75) under the crossed polarizer and analyzer.2.6. Measurement of the lipid oxidation products
For the lipid oxidation studies, all the vials were stored in a close box and stayed in a 45 °C incubator room in the dark for up to 11 days, while a short storage time may be reported when the oxidative kinetic went to the exponential phase. Lipid hydroperoxides were measured as the primary oxidation product using a method adapted from Shantha and Decker.15 The secondary oxidation product marker propanal was monitored using a GC-17A Shimadzu gas chromatograph bundled with an AOC-5000 autosampler (Shimadzu, Kyoto, Japan). Oil samples (1 mL) in 10 mL capped GC glass vials were preheated at 45 °C for 15 min in an AOC-5000 autosampler heating block. A 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane solid-phase microextraction (SPME) fiber needle (Supelco, Bellefonte, PA) was injected into the vial absorbing volatiles for 2 min, and then was transferred to the injector port (250 °C) for 3 min. Split mode was selected at the ratio of at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for the injection port. Volatiles were identified on a Supelco 30 m × 0.32 mm Equity DB-1 column with a 1 μm film thickness at a constant 65 °C for 10 min. The carrier gas was helium and the flow rate was 15.0 mL min−1. A flame ionization detector (FID) was set at a temperature of 250 °C. The concentration of propanal was calculated from its peak areas using a standard curve prepared from an authentic standard. The lag phase is defined as the time required to observe a sudden increase of hydroperoxides and propanal formation.
5 for the injection port. Volatiles were identified on a Supelco 30 m × 0.32 mm Equity DB-1 column with a 1 μm film thickness at a constant 65 °C for 10 min. The carrier gas was helium and the flow rate was 15.0 mL min−1. A flame ionization detector (FID) was set at a temperature of 250 °C. The concentration of propanal was calculated from its peak areas using a standard curve prepared from an authentic standard. The lag phase is defined as the time required to observe a sudden increase of hydroperoxides and propanal formation.
2.7. Statistical analysis
All the experiments were carried out in either duplicate or triplicate using freshly prepared samples. The results are reported as the calculated mean and standard deviation.3. Results and discussion
3.1. Formation and thermal properties of gelled algae oil
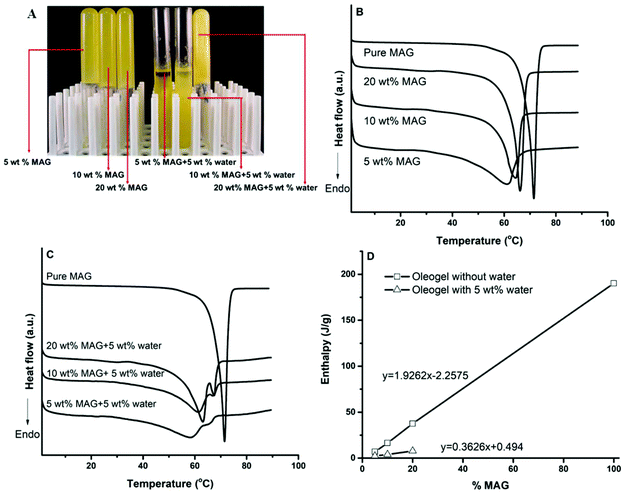
We first demonstrated the formation of gelled algae oil developed by MAG and analyzed the thermal behavior of algae oil oleogel since the concentration of the gelator could affect the thermal properties of the oleogel. Based on our preliminary study, monoacylglycerol used in this study was identified as an excellent gelator that could solidify algae oil for oleogel at concentrations as low as 3 wt%. However, the presence of 5 wt% water required a minimum of 20 wt% of MAG to solidify the algae oil (Fig. 1A). Phase separation and oiling-off were observed after 24 h storage at 4 °C as a lower concentration of MAG (5 and 10 wt%) was introduced. This was not surprising since the crystallization of MAG when in contact with water involves polymorphic transformation from sub α-gel (Lα) to α-gel, and even the formation of coagel (β-crystals in water), which gives rise to the loss of its oil binding capacity.16 This was confirmed by our observation that the gelled algae oil was formed by adding 10 wt% of MAG in the presence of 5 wt% water; however, the oil was gradually released during 7 days storage. Due to the fact that both a low and high amount of MAG (5–20 wt%) was used, we herein defined the solidified algae oil as gelled algae oil instead of oleogel.The thermal behavior of the gelled algae oil developed with various concentration of MAG in the absence (Fig. 1B) and presence of 5 wt% water after 7 days storage (Fig. 1C) was investigated using DSC. As can be observed in Fig. 1B, pure MAG owned the highest peak melting temperature (Tm) and enthalpy of melting (ΔHm), being 71.48 °C and 190.2 J g−1, respectively, implying a high purity.17 The melting temperature of gelled algae oil shifted to lower temperatures as compared with pure MAG, with DSC heating thermographs of the gelled algae oil exhibiting a single peak. An elevated peak melting temperature (Tm) was displayed with an increase in the MAG concentration in the gelled algae oil composition, with Tm being elevated from 61.50 °C to 66.11 °C for 5 to 20 wt% MAG. The enthalpy of melting (ΔHm) increased linearly with the increase in MAG concentration, with ΔHm increasing from 6.94 to 37.51 J g−1 for 5 to 20 wt% MAG (Fig. 1D). A high concentration of MAG increased the effective volume in algae oil that they presented as well as apparently assisting the formation and growth of the MAG crystalline network upon cooling, thereby yielding a much greater quantity of crystallized matrix in the gelled algae oil system.18
The incorporation of 5 wt% water exhibited significantly different thermal behaviors than for anhydrous systems (gelled algae oil without water), with two peak profiles in the heating thermograph being revealed, indicating two major thermal events (Fig. 1C). A broad preponderant peak occurred in the low temperature region and the melting temperature of each system in this region was slightly lower; whereas, the second thermal event took place in the high temperature region and the melting temperature was higher than that for anhydrous systems. Similar results were obtained by Alfutimie et al., who systematically investigated the phase behavior of mixed MAG in water system.19 They postulated that the first endothermic peak belonged to the melting of the mixture system, and the lower melting temperature could be attributed to the hydration of the MAG head group under the presence of water. The second endothermic peak corresponded to the formation of a new mode of structure upon further heating.20 Meanwhile, a linear relationship between the enthalpy of melting (ΔHm) and the MAG concentration was recorded. Nevertheless, the presence of water decreased the enthalpy of each system dramatically and the slope was ∼5 times lower than that of anhydrous systems, suggesting a lower thermodynamic stability (Fig. 1D).
3.2. Crystal morphology
In the next step, the morphology of the gelled algae oil fabricated by 20 wt% of MAG upon heating was characterized using temperature-controlled microscopy (Fig. 2). More specifically we investigated whether the presence of water (5 wt%) would significantly impact the microstructure of the gelled algae oil. For the anhydrous system (Fig. 2A), the black image was recorded at 25 °C and was due to the extremely dense and tight structure of oleogel, which did not allow light to pass through the sample. As the temperature was raised to 40 °C, birefringence and a denser crystalline structure could easily been seen because of the two indices of refraction from the fat solid crystals.21 Such a gelled algae oil with a dense crystalline network formed by a high concentration of MAG (20 wt%) differentiated them very well with oleogel structures formed by low concentrations of gelators (<10 wt%). Distinct needle-like crystals with the length ranging between 10 and 20 μm, appeared when further increasing the temperature to 50 °C, which was in good agreement with the results from previous studies.22 A partial melting of the needle-like crystals was exhibited through a transformation of the solid state to the liquid crystal state as the temperature increased to 55 °C (from DSC data, beginning at 50 °C). The disappearance of needle-like crystals and the formation of regionally dispersed grain-like crystals occurred as the temperature went up to 60 °C. This again was caused by the melting of the MAG crystalline network formed by the lower melting temperature fractions of MAG, thus losing the capacity to entrap algae oil under such temperature. To our surprise, instead of a complete melting of the crystals, striking spherical droplets were visible in the continuous algae oil phase upon heating to 65 °C. The feasible explanation for this involves a reorientation and rearrangement of the high-melting fractions of MAG before reaching their melting point.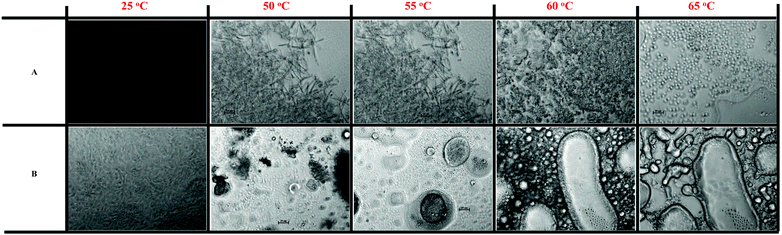 | ||
| Fig. 2 Polarized light microscopy images of gelled algae oil prepared consisting of 20 wt% monoglycerides and (A) 0 wt% or (B) 5 wt% water under different temperatures (scale bar = 20 μm). | ||
In contrast, the appearance of MAG crystalline was significantly different under the presence of 5 wt% water (Fig. 2B). Smaller fibrous crystals were clearly observed at 25 °C denoting the less dense nature of the structures. These fibrous structures were reported to create stronger crystalline matrices at the relatively low concentration.22 However, this was not the case in gelled algae oil when water was introduced, putatively because of the hydration of the polar head group of MAG, which then cannot mesh very well, and the efficiency of the inter-crystalline interface to entrap liquid oil. The fibrous crystalline structure disappeared with a concomitant change in the solid-like character as the temperature was increased to 50 °C (from DSC data, beginning at 42 °C). Meanwhile, water started to coalesce at this temperature, as evidenced by the occurrence of a dark region. Further heating the sample to 55 °C resulted in the formation of small water droplets, stabilized by the hydrophobic MAG due to its emulsification properties. Binks et al. observed a similar phenomenon and corroborated that hydrophobic molecular species melt upon heating toward the upper end of the melting range and dissolve in oil acting as efficient stabilizers of water drops.23 Consistent with the second melting event on the DSC thermograph (Fig. 1C), two modes of structures were visualized with significant birefringence as the temperature increased to 60 °C. The coalesced water was encircled by bright rings arising from the interfacial crystallization of MAG, whereas their local surroundings consisted of individual and aggregated MAG crystals and spherulites. As stated by Rousseau et al., such characteristics is common in type I puckering network crystals derived from the direct solidification of high-melting, oil-tending surfactants at the oil–water interface and in the continuous phase.24,25 Not surprisingly, further heating the system to 65 °C led to the complete melting of the spherulite structures and the formation of remarkable ternary regions of water, algae oil, and MAG.
3.3. Oscillatory rheology
Finally, the influence of water addition (0 and 5 wt%) on the rheological properties of the gelled algae oil consisting of different amounts of MAG (5, 10, and 20 wt%) was evaluated through the complex modulus (G*) under isothermal (Fig. 3) and temperature ramping (Fig. 4) conditions. Based on Fig. 3, gelled algae oil from 5 wt% of MAG was very weak with a G* value of around 1000 Pa, most likely due to the lower solids content and weak MAG crystalline network. A gradual increment occurred with the increase in oscillation frequency, suggesting its high shear sensitivity and weak gel nature.26 The strength of the gelled algae oil increased drastically when the concentration of MAG was over 10 wt% and displayed a straight line in a frequency-independent manner. Considering that the strength of the gelled algae oil is determined by the network of crystallized MAG, the current results highlighted that the increase of the MAG content led to the formation of a more structured crystalline network with greatest stability toward high oscillation frequency. Nevertheless, the concentration of MAG required to form a strong crystalline network in algae oil was significantly higher than in other liquid oil systems.27 This is not unusual since it has been reported that the minimum concentration of gelator needed to develop a gelled oil depends on the fatty acid composition of the liquid oil.28 Base algae oil features the highest unsaturation among liquid oils that has ever been studied for a gelled oil, thus requiring the highest amount of gelator to form a strong MAG crystalline network.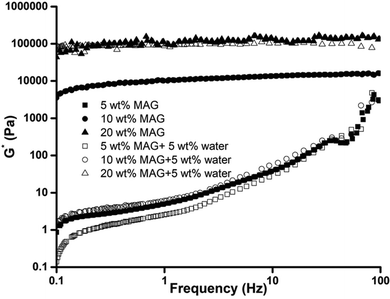 | ||
| Fig. 3 Frequency dependence of the complex modulus G* for gelled algae oil containing monoglycerides (MAG, 5, 10, 20 wt%) and water (0, 5 wt%) at 25 °C. | ||
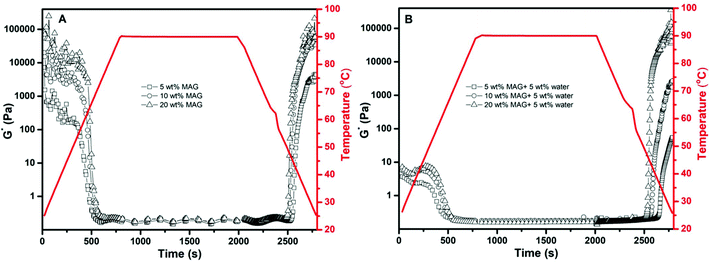 | ||
| Fig. 4 Development of complex modulus G* with time and temperature for (A) gelled algae oil containing monoacylglycerides (MAG, 5, 10, 20 wt%), and (B) water (5 wt%). | ||
The presence of water had an adverse impact on the viscoelastic properties of gelled algae oil. The viscoelasticity of the oleogel prepared by 5 and 10 wt% MAG had identical profiles, which can be described as a low G* value with frequency dependence. Such results can be confirmed by the image (Fig. 1A) showing that oiling-off and phase separation occurred after 7 days storage. The highest G* was exclusively recorded for gelled algae oil containing 20 wt% of MAG, with the value of G* being in the same magnitude as the anhydrous system. In other words, a high amount of gelator is able to maintain the strength of the gelled algae oil along with a limited amount of water. The addition of water did not alter the complex modulus of the system. Furthermore, the complex modulus was independent of the oscillation frequency with the range applied and behaved as a weak gel material.
The complex modulus (G*) of gelled algae oil as a function of heating and cooling shared some common characteristics independent of the water and MAG content (Fig. 4). First, all the systems could be reversibly altered between the semi-solid gel and liquid dispersion by changing the temperature, indicating their thermal reversibility. Such a property is beneficial from the viewpoint of food formulations when a thermo-reversible nature is desired. Second, the non-superimposable complex modulus curve upon heating and cooling suggested a hysteresis effect during the gel-to-liquid dispersion transition, which is also common in other oleogel systems.29 The concentration of MAG had a limited impact on the hysteresis effect in the gelled algae oil system in the absence of water (Fig. 4A). While complex modulus characterization yields similar results, they also generated some interesting results. In particular, the complex modulus curve of gelled algae oil in the absence of water revealed a continuous decrease during heating and a continuous increase during cooling. Such behavior, as pointed out by Davidovich-Pinhas et al., suggested the gelation of algae oil does not induce the formation of an organized secondary structure.30 However, different complex modulus curves have emerged in algae oil oleogel with 5 wt% water presented. An inflection point, as described by a rapid decline in the complex modulus followed by a considerable increase upon heating, was recorded (Fig. 4B). The greater increase in the complex modulus was correlated to the higher MAG concentration (>10 wt%). This again coincided with the previous DSC and morphology results showing that a new mode of structure was formed when introducing water into the MAG–algae oil system.
3.4. Impact of MAG concentration on the oxidative stability of gelled algae oil
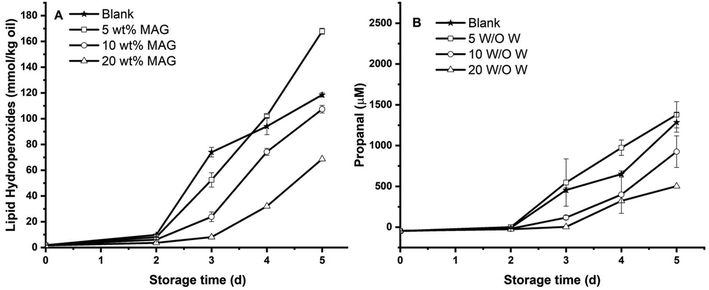
One goal of this study was to understand the role of the MAG crystalline network on the oxidative stability of gelled algae oil. In this section, the impact of different levels of MAG (5, 10, 20 wt%) on lipid oxidation of gelled algae oil in the absence of water was investigated by measuring the formation of lipid hydroperoxides (LHs) and headspace propanal during storage at 45 °C (Fig. 5).The formation of LHs in gelled algae oil prepared by a relatively lower amount of MAG (5 wt%) increased dramatically after 2 days storage at 45 °C, with no significant difference from liquid algae oil (Fig. 5A). The higher concentration of MAG (10 wt%) was able to produce less LHs after 3 days storage compared with the system with the lowest MAG. However, these differences were not believed to be important for the oxidative stability of food systems since both had already gone through an exponential phase of oxidation on day 3. The gelled algae oil with the highest MAG concentration (20 wt%) delayed lipid oxidation and an extended lag phase to 3 days in terms of the formation of LHs. The formation of propanal in gelled algae oil with different MAG concentration followed similar trends, and the lag phase increased with increasing the MAG to 10 wt%. However, a further increase of MAG to 20 wt% did not contribute to the improved oxidative stability and only retained a 3-day lag phase (Fig. 5B). This result indicated that the addition of MAG (>10 wt%) could potentially prolong the lag phase of algae oil oleogel to a certain extent. A previous study on the oxidative stability of cod oil oleogel formed by MAG concluded that the presence of MAG was ineffective at affecting the formation of LHs but appeared to constitute a hurdle against the development of propanal.5 This study only covered the low concentration range of MAG (<9 wt%) and the results were in agreement with our findings for oleogel formed by 5 wt% MAG. However, higher concentration of MAG exhibited protective activity against gelled algae oil oxidation. In addition, our previous study revealed that MAG itself acted as neither an antioxidant nor prooxidant in stripped soybean oil oxidation.12 The lipid oxidation between environmental oxygen and unsaturated fatty acid, resulting in the formation of primary (e.g., LHs) and secondary oxidation products (e.g., volatiles), is a diffusion limited reactions.31 In other works, a trace amount of oxygen could trigger free radical chain reactions. The antioxidative effect of MAG at higher concentration (>10 wt%) in algae oil oleogel could presumably be due to the formation of an extremely dense MAG crystalline network, acting as a physical barrier that could restrain the reaction between the unpaired electrons of oxygen and unsaturated fatty acid radicals produced during the gelling process or storage.
3.5. Impact of water addition on the oxidative stability of gelled algae oil
In this section, the presence of water (5 wt%) on the lipid oxidation of gelled algae oil solidified by MAG (5, 10, 20 wt%) was investigated by measuring the formation of lipid hydroperoxides and headspace propanal during storage at 45 °C (Fig. 5).Similar to liquid algae oil, the lag phase of LH formation for all gelled algae oil samples was only 2 days. It was also independent of MAG concentration, although the absolute amount of LHs was lower as a higher amount of MAG was applied (Fig. 6A). Likewise, the addition of water demonstrated an identical impact on the formation of propanal as it had for LHs, which gave rise to a 2-day lag phase for all the systems (Fig. 6B). These findings indicated that the protective effect of MAG arising at higher concentration (>10 wt%) on the anhydrous system was completely eliminated when 5 wt% water was introduced. As displayed in Fig. 1A, the addition of water in the algae oil–MAG binary system led to the formation of two different systems. At lower MAG concentration (5, 10 wt%), oiling-off and phase separation were observed. Therefore, it was not surprising that the oxidative stability of such a system would be similar to that of algae oil. At a higher MAG concentration (20 wt%), a solid-like system was built up. The previous physical characterizations also demonstrated the existence of an ordered structure other than just the primary MAG crystalline network in the anhydrous system. As such, the strong crystalline network formed exclusively at high MAG concentration would not be in favor of the interaction of oxygen and radicals even though water was added, thus suppressing the formation of both oxidation markers. However, the unchanged lag phase of the gelled algae oil in the presence of water remains unexplained.
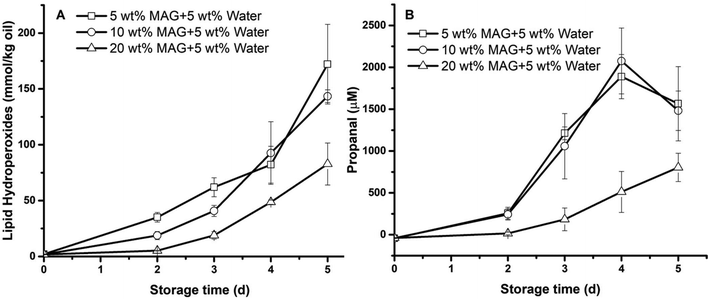 | ||
| Fig. 6 Formation of (A) lipid hydroperoxides, (B) propanal in gelled algae oil containing monoacylglycerides (MAG, 5, 10, 20 wt%) and 5 wt% water at 45 °C. Some error bars are within data points. | ||
3.6. Impact of hydrophilic antioxidants on the oxidative stability of gelled algae oil
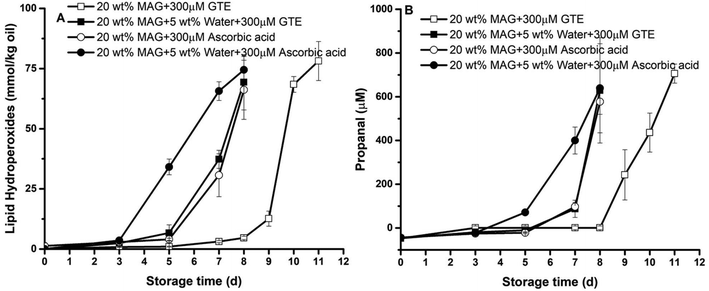
Although a higher concentration of MAG was able to slow down the lipid oxidation of algae oil oxidation, such an ability was still insufficient since it could only extend the lag phase from 2 to 3 days. Therefore, another goal of this study was to develop a strategy of using an antioxidant to further protect gelled algae oil against oxidation during the harsh gelling process and storage. A previous study found that some hydrophilic natural antioxidants, such as green tea extract (GTE) and ascorbic acid (AA) at 300 μM, can effectively suspend the lipid oxidation of base algae oil and water-in-algae oil emulsion.13 In this section, we further examined the efficacy of those two hydrophilic antioxidants on the oxidative stability of gelled algae oil systems in the absence and presence of 5 wt% water. For better comparison, 20 wt% of MAG was used since physically stable systems can only be formed in both systems under this concentration. The formation of the primary oxidation marker lipid hydroperoxides (LHs) and secondary oxidation marker propanal was assessed in such systems during storage at 45 °C (Fig. 7).In general, the incorporation of hydrophilic antioxidants delayed the oxidation of gelled algae oil. In the absence of water, the lag phase of both the LHs and propanal formation for gelled algae oil containing 300 μM AA was 5 days, being 3 days and 1 day longer than the same of base algae oil without or with the same amount of AA, respectively.13 When the same concentration of GTE was incorporated into the gelled algae oil, the antioxidative behavior was appreciably boosted by generating an 8-day lag phase in both LHs and propanal formation. We extrapolated that the crystalline network formed by MAG in the gelled algae oil may play a big role in synergizing with the hydrophilic antioxidants to retard lipid oxidation. However, different antioxidative effectiveness was observed between AA and GTE, which was surprising since our previous study found that the antioxidative effectiveness of 300 μM AA and GTE tied in with each other and only extended the lag phase of base algae oil to 4 days.13 Both GTE and AA are free radical scavengers that can quench the radicals initiated upon lipid oxidation. In addition, AA can act as transition metal chelator and singlet oxygen quencher to slow down the formation of secondary oxidation products.32 The reason why GTE was superior to AA in protecting the gelled algae oil against oxidation is unknown at this stage. It is less likely to be because of the degradation of AA during the gelling process at 75 °C since AA has excellent thermal stability and may undergo degradation only at 190 °C.33 One possible explanation thus was due to the crystallization of partially soluble AA during the gelling process upon cooling from 75 °C to 4 °C, which accounts for its low antioxidative activity. This might also be the reason why ascorbyl palmitate, the esterified ascorbic acid with palmitic acid, is widely used in bulk and frying oil to enhance the oxidative stability.34,35
Interestingly, the addition of 5 wt% water again attenuated the antioxidative effectiveness of both hydrophilic antioxidants on the gelled algae oil oxidation. In particular, the incorporation of 300 μM AA extended the lag phase of LHs and propanal accumulation to only 3 days, whereas the same concentrations of GTE generated a 5-day lag phase. Compared to the antioxidative activity of AA in base algae oil, which contributed to the 4-day lag phase, the presence of water seemed to suppress the effectiveness of AA. One possible explanation for this was that the location of AA and GTE in the anhydrous gelled algae oil was different than in the system with the water present. Upon the addition of water, hydrophilic antioxidants are concentrated in the small region where water exist. The oxidation of gelled algae oil would be similar to bulk oil, where the oxidation occurs at the oil/air interface. The previous physical characterization showed that the MAG crystalline network will refrain water from moving to the interface. Therefore, the oxidation of algae oil in the gelled system cannot be effectively prevented. Still, further research is required to elucidate the mechanism by assessing the location of antioxidants in a gelled oil system in the presence of water.
Based on the physical characterization and the oxidative stability of gelled algae oil, the synergistic effect of the MAG crystalline network and hydrophilic antioxidants to inhibit gelled algae oil oxidation is proposed and illustrated in Fig. 8. The addition of a low concentration of MAG (5 wt%) was able to form a relatively weak and larger crystalline network to entrap big algae oil droplets. When increasing the MAG to 10 and 20 wt%, a dense crystalline network with fine MAG crystalline is developed. The algae oil is well dispersed in such a crystalline network, which can create a physical barrier to prevent attack from oxygen, thus yielding higher oxidative stability. The addition of antioxidants can further create a thermodynamic barrier to quench the free radicals generated during the storage. Such strategies can be considered as a novel technique to effectively prevent lipid oxidation in food systems.36 As 5 wt% water was introduced, the physically stable and strong MAG crystalline network could be formed only when 20 wt% MAG was included. Besides, an ordered structure other than MAG crystalline network is formed as evidenced by DSC, morphology, and viscoelastic study. Owning to the surface activity nature of MAG, the addition of water can form α-gel with MAG.37 Such a structure is unstable at lower MAG concentrations (5, 10 wt%), under which algae oil cannot be effectively entrapped and protected. In consequence, the oxidation of such a system would not be different than for algae oil. When the concentration of MAG (20 wt%) is high enough, the order structure formed between MAG and water can be encompassed and underpinned by the crystalline network created by the extra amount of MAG, which can still diminish the movement of oxygen. However, hydrophilic antioxidants, such as AA and GTE, are more favorable in the water phase than in algae oil. Therefore, it is reasonable that the existence of water could countervail the efficacy of the hydrophilic antioxidants.
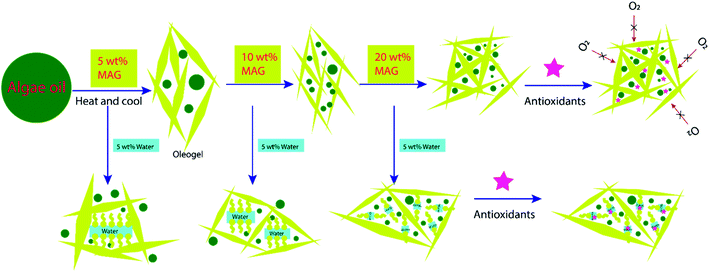 | ||
| Fig. 8 Postulated synergestic effect of the monoglycerides crystalline network and hydrophilic antioxidants on inhibiting algae oil oxidation. | ||
4. Conclusions
This research demonstrated that algae oil can be structured to a semi-solid state using monoacylglycerol. The concentration of monoacylglycerol had a remarkable influence on the physical properties of the gelled algae oil, with the melting temperature (Tm), enthalpy of melting (ΔHm), and gel strength increasing upon the increase in monoacylglycerol concentration, indicating the formation of a strong MAG crystalline network. The addition of 5 wt% water had interactive impacts on the role of monoacylglycerol in entrapping algae oil by forming not only α-gel but also an ordered structure. Therefore, only a higher concentration of monoacylglycerol (20 wt%) could form a strong crystalline network to physically stabilize the algae oil. A lipid oxidation study of the gelled system revealed that physical barriers created by the MAG crystalline network could prevent the oxidation of algae oil to a lesser extent. By combining the physical barrier with a thermodynamic barrier initiated by the addition of green tea extract, a novel hurdle technique was developed to effectively retard the oxidation of gelled algae oil by 8 days during storage at 45 °C, resulting in a 4-fold improvement in the lag phase of base algae oil. However, hydrophilic antioxidants with a higher tendency to crystalize during the heating–cooling of oil during the gelling process may not be appropriate candidates to prevent gelled oil oxidation, as was the case for ascorbic acid. There findings may provide a convenient means for the food industry to incorporate more n-3 LC-PUFA into food products with enhanced oxidative stability by means of a gelling process.Conflicts of interest
There are no conflicts to declare.References
- H. Yu, K. Shi, D. Liu and Q. Huang, Food Chem., 2012, 131, 48–54 CrossRef CAS.
- M. D. Bin Sintang, S. Danthine, A. Brown, D. Van de Walle, A. R. Patel, I. Tavernier, T. Rimaux and K. Dewettinck, Food Res. Int., 2017, 100, 832–840 CrossRef CAS PubMed.
- A. J. Gravelle, S. Barbut and A. G. Marangoni, Food Res. Int., 2012, 48, 578–583 CrossRef CAS.
- A. J. Martins, A. A. Vicente, R. L. Cunha and M. A. Cerqueira, Food Funct., 2018, 9, 758–773 RSC.
- S. Da Pieve, S. Calligaris, A. Panozzo, G. Arrighetti and M. C. Nicoli, Food Res. Int., 2011, 44, 2978–2983 CrossRef CAS.
- M. Öğütcü, N. Arifoğlu and E. Yılmaz, Int. J. Food Sci. Technol., 2015, 50, 404–412 CrossRef.
- M. Öǧütcü, R. Temizkan, N. Arifoǧlu and E. Yılmaz, J. Oleo Sci., 2015, 64, 713–720 CrossRef.
- B. R. Yi, M. J. Kim, S. Y. Lee and J. H. Lee, Food Sci. Biotechnol., 2017, 26, 79–87 CrossRef CAS.
- J. Lim, H. S. Hwang and S. Lee, Appl. Biol. Chem., 2017, 60, 17–22 CrossRef CAS.
- S. Da Pieve, S. Calligaris, E. Co, M. C. Nicoli and A. G. Marangoni, Food Biophys., 2010, 5, 211–217 CrossRef.
- A. López-Martínez, J. A. Morales-Rueda, E. Dibildox-Alvarado, M. A. Charó-Alonso, A. G. Marangoni and J. F. Toro-Vazquez, Food Res. Int., 2014, 64, 946–957 CrossRef PubMed.
- B. Chen, D. J. Mcclements and E. A. Decker, Food Chem., 2014, 142, 365–372 CrossRef CAS PubMed.
- B. Chen, J. Rao, Y. Ding, D. J. McClements and E. A. Decker, Food Res. Int., 2016, 85, 162–169 CrossRef CAS PubMed.
- J. Yin, E. M. Becker, M. L. Andersen and L. H. Skibsted, Food Chem., 2012, 135, 2195–2202 CrossRef CAS.
- N. C. Shantha and E. A. Decker, J. AOAC Int., 1994, 77, 421–424 CAS.
- A. Sein, J. A. Verheij and W. G. M. Agterof, J. Colloid Interface Sci., 2002, 249, 412–422 CrossRef CAS PubMed.
- M. Öğütcü and E. Yılmaz, J. Appl. Polym. Sci., 2015, 132, 1–8 CrossRef.
- J. E. Martín-Alfonso and C. Valencia, Tribol. Int., 2015, 90, 426–434 CrossRef.
- A. Alfutimie, R. Curtis and G. J. T. Tiddy, Colloids Surf., A, 2015, 465, 99–105 CrossRef CAS.
- Y. Cegla-Nemirovsky, A. Aserin and N. Garti, J. Am. Oil Chem. Soc., 2015, 92, 439–447 CrossRef CAS.
- A. Zetzl, M. Ollivon and A. Marangoni, Cryst. Growth Des., 2009, 9, 3928–3933 CrossRef CAS.
- A. I. Blake, E. D. Co and A. G. Marangoni, J. Am. Oil Chem. Soc., 2014, 91, 885–903 CrossRef CAS.
- B. P. Binks and A. Rocher, J. Colloid Interface Sci., 2009, 335, 94–104 CrossRef CAS PubMed.
- D. Rousseau, Curr. Opin. Colloid Interface Sci., 2013, 18, 283–291 CrossRef CAS.
- S. Ghosh and D. Rousseau, Cryst. Growth Des., 2012, 12, 4944–4954 CrossRef CAS.
- M. D. Bin Sintang, T. Rimaux, D. Van de Walle, K. Dewettinck and A. R. Patel, Eur. J. Lipid Sci. Technol., 2017, 119, 1–14 CrossRef.
- A. López-Martínez, M. A. Charó-Alonso, A. G. Marangoni and J. F. Toro-Vazquez, Food Res. Int., 2015, 72, 37–46 CrossRef.
- A. K. Zetzl, A. G. Marangoni and S. Barbut, Food Funct., 2012, 3, 327–337 RSC.
- J. F. Toro-Vazquez, J. A. Morales-Rueda, E. Dibildox-Alvarado, M. Charó-Alonso, M. Alonzo-Macias and M. M. González-Chávez, J. Am. Oil Chem. Soc., 2007, 84, 989–1000 CrossRef CAS.
- M. Davidovich-Pinhas, S. Barbut and A. G. Marangoni, Carbohydr. Polym., 2015, 117, 869–878 CrossRef CAS PubMed.
- D. R. Johnson and E. A. Decker, Annu. Rev. Food Sci. Technol., 2015, 6, 171–190 CrossRef CAS PubMed.
- S. Uluata, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2015, 63, 1819–1824 CrossRef CAS.
- S. Y. Reda, Food Sci. Technol., 2011, 31, 475–480 CrossRef.
- A. Satyanaryana, N. Giridhar, G. J. Joshi and D. G. Rao, J. Food Lipids, 2000, 7, 1–10 CrossRef.
- R. Upadhyay, S. Sehwag and H. Niwas Mishra, Food Chem., 2017, 218, 496–504 CrossRef CAS PubMed.
- E. A. Decker, D. J. McClements, C. Bourlieu-Lacanal, E. Durand, M. C. Figueroa-Espinoza, J. Lecomte and P. Villeneuve, Trends Food Sci. Technol., 2017, 67, 183–194 CrossRef CAS.
- C. H. Chen and E. M. Terentjev, Langmuir, 2010, 26, 3095–3105 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |