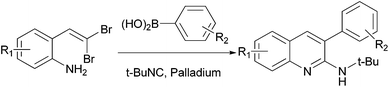
Synthesis of 3-aryl-2-aminoquinolines: palladium-catalyzed cascade reactions of gem-dibromovinylanilines with tert-butyl isocyanide and arylboronic acids†
Langxi Hu‡
a,
Weijun Gui‡a,
Zichen Liub and
Baishan Jiang *a
*a
aInstitute of Chemical Biology, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, 190 Kaiyuan Avenue, Guangzhou 510530, China. E-mail: jiang_baishan@gibh.ac.cn; Fax: +86-20-3201-5209; Tel: +86-20-3201-5209
bShenzhen University Health Science Center, Shenzhen University, China
First published on 13th August 2014
Abstract
A three-component cascade reaction involving gem-dibromovinylanilines, tert-butyl isocyanide and arylboronic acids for the efficient synthesis of 3-aryl-2-aminoquinolines has been developed. The reaction proceeds through palladium-catalyzed isocyanide insertion, intramolecular cyclization of gem-dibromovinylanilines followed by Suzuki coupling with arylboronic acids, and the corresponding products were obtained in good to excellent isolated yields.
The quinoline nucleus is a privileged scaffold that exists in a large number of natural products and synthetic drugs with varied bioactivities.1 In particular, the 2-aminoquinoline derivatives have been frequently studied during the past decades because of their pharmacological potential covering a range of possible applications, including anti-Alzheimer's,2 anti-hypertensive3 and anti-cancer4 activities. While a great number of synthetic methods have been developed for quinolines synthesis,5 only few examples have been reported for the synthesis of 2-aminoqunolines, including (a) Buchwald–Hartwig aminations of 2-halo-quinolines,6a (b) palladium-catalyzed oxidation cyclizations of 2-ethynylanilines with isocyanides,6b (c) thermal rearrangement reactions of aldehydes, secondary amines and aryl azides,6c and (d) direct amination of quinoline N-oxides.6d,6e The major drawbacks of the existing methods are their complicated multistep procedures, limited availability of substrates or high reaction temperatures, which usually hinder them from constructing a large number of structural derivatives of 2-aminoquinolines. Therefore, development of general and efficient strategies for the preparation of 2-aminoquinolines, especially via multi-component cascade reactions starting from readily available sources, is still highly desirable.
gem-Dibromovinylanilines are valuable synthetic intermediates in organic synthesis because they are highly reactive and can be easily obtained from inexpensive aldehydes.7 In recent years, Lautens and other groups have reported a number of elegant strategies for the synthesis of various indole derivatives from gem-dibromovinylanilines via palladium and/or copper-catalyzed cascade cross-coupling reactions, such as C–N/C–N,8 C–N/C–C,9 C–N/C–H,10 C–N/C–P,9f C–N/carbonylation,11a and C–N/carbonylation/C–C reactions.11b However, to the best of our knowledge, the construction of 2-aminoquinoline scaffolds from gem-dibromovinylanilines remains scarce.
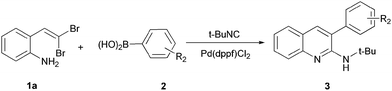
Compared to the traditional stepwise synthesis, the cascade (tandem) reaction is the most facile and economic synthetic approach. It enhances the reaction efficiency and avoids the tedious step-by-step separations and purifications of intermediates.12 In our continuous studies of 1,1-dibromoolefins13 and our interests in looking for 2-aminoquinoline anti-cancer agents, we disclose herein an efficient synthesis of 3-aryl-2-aminoquinoline derivatives via cascade palladium-catalyzed isocyanide insertion, intramolecular cyclization and Suzuki coupling of gem-dibromovinylanilines (Scheme 1). The reaction involves gem-dibromovinylanilines, arylboronic acids, and tert-butyl isocyanide.
Our study commenced with the treatment of gem-dibromovinylaniline (1a), phenylboronic acid (2a), and t-butyl isocyanide in the presence of Pd(dppf)Cl2 and K2CO3 in toluene for 6 h at 100 °C. Gratifyingly, the expected quinoline product 3a was obtained in 47% yield (Table 1, entry 1). To improve the yield, screening of the reaction conditions was then carried out. Firstly, by using Pd(dppf)Cl2 as catalyst, the effect of different solvents including toluene, CH3CN, DMF, THF and 1,4-dioxane was studied (Table 1, entries 1–5). Among them, 1,4-dioxane gave 3a in the highest yield (Table 1, entry 5). After screening on a series of palladium catalysts including Pd(dppf)Cl2, Pd(PPh3)2Cl2, Pd(acac)2, PdCl2, Pd(OAc)2 and Pd(TFA)2 (Table 1, entries 5–10), Pd(dppf)Cl2 turned out to be the best choice (Table 1, entry 5). Among the tested bases, Cs2CO3 gave 3a the highest yield (Table 1, entries 11–14). In addition, elevating or lowering reaction temperature resulted in decreased yields (Table 1, entries 15–16).
| Entry | Catalyst | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), t-BuNC (0.22 mmol), 2a (0.22 mmol), base (0.44 mmol), catalyst (0.01 mmol), sealed tube, 100 °C, 8 h.b Isolated yields.c 120 °C.d 80 °C. | ||||
| 1 | Pd(dppf)Cl2 | Toluene | K2CO3 | 47 |
| 2 | Pd(dppf)Cl2 | CH3CN | K2CO3 | 21 |
| 3 | Pd(dppf)Cl2 | DMF | K2CO3 | 40 |
| 4 | Pd(dppf)Cl2 | THF | K2CO3 | 58 |
| 5 | Pd(dppf)Cl2 | Dioxane | K2CO3 | 70 |
| 6 | Pd(PPh3)2Cl2 | Dioxane | K2CO3 | 46 |
| 7 | Pd(acac)2 | Dioxane | K2CO3 | 35 |
| 8 | PdCl2 | Dioxane | K2CO3 | 14 |
| 9 | Pd(OAc)2 | Dioxane | K2CO3 | 31 |
| 10 | Pd(TFA)2 | Dioxane | K2CO3 | 35 |
| 11 | Pd(dppf)Cl2 | Dioxane | K3PO4 | 63 |
| 12 | Pd(dppf)Cl2 | Dioxane | Na2CO3 | 61 |
| 13 | Pd(dppf)Cl2 | Dioxane | NaOH | 46 |
| 14 | Pd(dppf)Cl2 | Dioxane | Cs2CO3 | 78 |
| 15 | Pd(dppf)Cl2 | Dioxane | Cs2CO3 | 57c |
| 16 | Pd(dppf)Cl2 | Dioxane | Cs2CO3 | 66d |
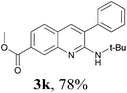
With the optimized reaction condition in hand (Table 1, entry 14), the scope of the reaction for different gem-dibromovinylanilines was investigated firstly, and the results are summarized in Table 2. Overall, we were pleased with the generality of our protocol. As showed in Table 2, the expected products 3 were obtained in good isolated yields (3b–l). Several functional groups were well tolerated including chloro (3b, 3c), fluro (3d, 3e). It is obvious that the electronic effects of the substituted groups on the gem-dibromovinylanilines did not affect the efficacy of the cascade reaction (3f–l).
| a Reaction conditions: 1 (0.2 mmol), RNC (0.22 mmol), 2a (0.22 mmol), Cs2CO3 (0.44 mmol), Pd(dppf)Cl2 (0.01 mmol), 1,4-dioxane (2.0 mL), sealed tube, 100 °C, 8 h. | ||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
The influence of the substituents in the arylboronic acids was also evaluated and the results are presented in Table 3. In all cases, the desired products were obtained in good to excellent isolated yields. Halogen substitutes, including chloro and fluro, on the aromatic ring of boronic acid were tolerated (3m, 3n). Electron-donating substrates (3o–r) afforded the quinoline products 3 in better yields compared with the electron-withdrawing ones (3s–v). Other heteroaryl boronic acid like 3-thienylboronic acid proved to be an efficient partner of the cascade reaction providing the desired product 3w in good yield. Isocyanides other than tert-butyl isocyanide were also applied in this process, however limited success was achieved (3x, 3y).
| a Reaction conditions: 1a (0.2 mmol), t-BuNC (0.22 mmol), 2 (0.22 mmol), Cs2CO3 (0.44 mmol), Pd(dppf)Cl2 (0.01 mmol), 1,4-dioxane (2.0 mL), sealed tube, 100 °C, 8 h. | ||||
|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
||
The synthesized 3-aryl-2-aminoquinolines are very useful intermediates as the t-butyl group can be easily removed according to a method reported in the literature,14 releasing a free amine group which may then be subject to various further transformations.15 For example, when 3c was heated to reflux in trifluoroacetic acid, the desired 2-aminoquinoline 4c was obtained in 83% isolated yield (Scheme 2).
To gain some insights into the mechanism of the reaction, several control experiments were conducted as shown in Scheme 3. When 1a and t-BuNC (1.1 equiv.) were treated under the optimized reaction conditions, 86% of the 3-bromo quinoline 5 was obtained 3 h later. After 1a was consumed monitored by TLC, phenylboronic acid (1.1 equiv.) was added directly to the cooled reaction mixture and heated to 100 °C for 6 h, 82% of the desired product 3a was obtained. When the three components were carried out under the optimized conditions for 0.5 h, 81% of the newly formed products was 3-bromo quinoline 5 (determined by LC-MS). These experimental results revealed that this reaction proceeded through the migratory insertion of isocyanide, and then the Suzuki reaction.
Based on literature reports16 and our experimental observations, a plausible mechanism was proposed (Scheme 4). The isocyanide-complexed Pd species A was firstly formed. Then, oxidative addition of cis-bromovinyl of 1a to the Pd species A formed the vinylpalladium complex B via ortho-aniline assistance. Subsequent migratory insertion of t-butyl isocyanide to B gave intermediate C, followed by intramolecular nucleophilic attack of the aniline to provide intermediate D and hydrogen bromide. Reductive elimination of D afforded intermediate E, generating the Pd(0) catalyst. E was then subjected to Suzuki coupling reaction with phenylboronic acid giving the final product 3a.
Conclusions
In conclusion, we have developed a novel and efficient procedure for the synthesis of 3-aryl-2-aminoquinolines via a palladium-catalyzed three-component cascade reaction of gem-dibromo-vinylanilines, tert-butyl isocyanide and arylboronic acids. The reaction proceeds through palladium-catalyzed isocyanide insertion, intramolecular cyclization of gem-dibromovinylanilines followed by Suzuki coupling with arylboronic acids, and the corresponding products were obtained in good to excellent isolated yields. Furthermore, the tert-butyl group of the synthesized 3-aryl-2-aminoquinolines can be easily removed, releasing a free amine group which could easily bring structural diversity at the C2 position of the quinoline core. Further studies on the detailed mechanism and synthetic applications are ongoing in our laboratory and will be reported in due course.Acknowledgements
This work was financially supported by Foundation of the Leading Talents of Guangdong Province (no. 1187000044).Notes and references
- (a) K. Andries, P. Verhasselt, J. Guillemont, H. W. H. Göhlmann, J.-M. Neefs, H. Winkler, J. V. Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. d. Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis and V. Jarlier, Science, 2005, 307, 223 CrossRef CAS PubMed; (b) B. D. Bax, P. F. Chan, D. S. Eggleston, A. Fosberry, D. R. Gentry, F. Gorrec, I. Giordano, M. M. Hann, A. Hennessy, M. Hibbs, J. Huang, E. Jones, J. Jones, K. K. Brown, C. J. Lewis, E. W. May, M. R. Saunders, O. Singh, C. E. Spitzfaden, C. Shen, A. Shillings, A. J. Theobald, A. Wohlkonig, N. D. Pearson and M. N. Gwynn, Nature, 2010, 466, 935 CrossRef PubMed.
- Y. Cheng, T. C. Judd, M. D. Bartberger, J. Brown, K. Chen, J. Robert, T. Fremeau, D. Hickman, S. A. Hitchcock, B. Jordan, V. Li, P. Lopez, S. W. Louie, Y. Luo, K. Michelsen, T. Nixey, T. S. Powers, C. Rattan, E. A. Sickmier, J. David, J. St. Jean, R. C. Wahl, P. H. Wen and S. Wood, J. Med. Chem., 2011, 54, 5836 CrossRef CAS PubMed.
- S. F. Campbell, J. D. Hardstone and M. J. Palmer, J. Med. Chem., 1988, 31, 1031 CrossRef CAS; R. L. Knight, D. R. Allen, H. L. Birch, G. A. Chapman, F. C. Galvin, L. A. Jopling, C. J. Lock, J. W. G. Meissner, D. A. Owen, G. Raphy, R. J. Watson and S. C. Williams, Bioorg. Med. Chem. Lett., 2008, 18, 629 CrossRef PubMed.
- S. R. Inglis, R. K. Jones, G. W. Booker and S. M. Pyke, Bioorg. Med. Chem. Lett., 2006, 16, 387 CrossRef CAS PubMed.
- For selected publications on quinoline synthesis, see: (a) J. Marco-Contelles, E. Pérez-Mayoral, A. Samadi, M. a. d. C. Carreiras and E. Soriano, Chem. Rev., 2009, 109, 2652 CrossRef CAS PubMed; (b) R. Yan, X. Liu, C. Pan, X. Zhou, X. Li, X. Kang and G. Huang, Org. Lett., 2013, 15, 4876 CrossRef CAS PubMed; (c) Y. Laras, V. Hugues, Y. Chandrasekaran, M. Blanchard-Desce, F. C. Acher and N. Pietrancosta, J. Org. Chem., 2012, 77, 8294 CrossRef CAS PubMed; (d) Y. Zhang, M. Wang, P. Li and L. Wang, Org. Lett., 2012, 14, 2206 CrossRef CAS PubMed; (e) S. Khong and O. Kwon, J. Org. Chem., 2012, 77, 8257 CrossRef CAS PubMed; (f) Y. C. Wu, L. Liu, H. J. Li, D. Wang and Y. J. Chen, J. Org. Chem., 2006, 71, 6592 CrossRef CAS PubMed.
- (a) V. Pavlovic, M. Petkovic, S. Popovic and V. Savic, Synth. Commun., 2009, 39, 4249 CrossRef CAS; (b) B. Liu, H. Gao, Y. Yu, W. Wu and H. Jiang, J. Org. Chem., 2013, 78, 10319 CrossRef CAS PubMed; (c) E. M. Beccalli, E. Erba, M. L. Gelmi and D. Pocar, J. Chem. Soc., Perkin Trans. 1, 1996, 1359 RSC; (d) C. Zhu, M. Yi, D. Wei, X. Chen, Y. Wu and X. Cui, Org. Lett., 2014, 16, 1840 CrossRef CAS PubMed; (e) G. Li, C. Jia and K. Sun, Org. Lett., 2013, 15, 5198 CrossRef CAS PubMed.
- G. Chelucci, Chem. Rev., 2012, 112, 1344 CrossRef CAS PubMed.
- J. Yuen, Y. Q. Fang and M. Lautens, Org. Lett., 2006, 8, 653 CrossRef CAS PubMed.
- (a) Y. Q. Fang, R. Karisch and M. Lautens, J. Org. Chem., 2007, 72, 1341 CrossRef CAS PubMed; (b) Y. Q. Fang, J. Yuen and M. Lautens, J. Org. Chem., 2007, 72, 5152 CrossRef CAS PubMed; (c) M. Nagamochi, Y. Q. Fang and M. Lautens, Org. Lett., 2007, 9, 2955 CrossRef CAS PubMed; (d) A. Fayol, Y. Q. Fang and M. Lautens, Org. Lett., 2006, 8, 4203 CrossRef CAS PubMed; (e) Y. Q. Fang and M. Lautens, Org. Lett., 2005, 7, 3549 CrossRef CAS PubMed; (f) Y. Q. Fang and M. Lautens, J. Org. Chem., 2008, 73, 538 CrossRef CAS PubMed; (g) S. Thielges, E. Meddah, P. Bisseret and J. Eustache, Tetrahedron Lett., 2004, 45, 907 CrossRef CAS PubMed.
- (a) W. Chen, M. Wang, P. Li and L. Wang, Tetrahedron Lett., 2011, 67, 5913 CrossRef CAS PubMed; (b) X. R. Qin, X. F. Cong, D. B. Zhao, J. S. You and J. B. Lan, Chem. Commun., 2011, 47, 5611 RSC; (c) Z. J. Wang, F. Yang, X. Lv and W. Bao, J. Org. Chem., 2011, 76, 967 CrossRef CAS PubMed; (d) Z. J. Wang, J. G. Yang, F. Yang and W. Bao, Org. Lett., 2010, 12, 3034 CrossRef CAS PubMed; (e) C. S. Bryan and M. Lautens, Org. Lett., 2008, 10, 4633 CrossRef CAS PubMed.
- (a) T. Vieira, L. A. Meaney, Y. L. Shi and H. Alper, Org. Lett., 2008, 10, 4899 CrossRef CAS PubMed; (b) M. Arthuis, R. Pontikis and J. C. Florent, Org. Lett., 2009, 11, 4608 CrossRef CAS PubMed.
- (a) Y. Liu and J. Wan, Org. Biomol. Chem., 2011, 9, 6873 RSC; (b) Y. Liu and J. Wan, Chem.–Asian J., 2012, 7, 1488 CrossRef CAS PubMed.
- (a) B. Jiang, L. Hu and W. Gui, RSC Adv., 2014, 4, 13850 RSC; (b) B. Jiang, K. Tao, W. Shen and J. Zhang, Tetrahedron Lett., 2010, 51, 6342 CrossRef CAS PubMed.
- K. D. Schleicher and T. F. Jamison, Org. Lett., 2007, 9, 875 CrossRef CAS PubMed.
- (a) J. Xie, X. Yuan, A. Abdukader, C. Zhu and J. Ma, Org. Lett., 2014, 16, 1768 CrossRef CAS PubMed; (b) C. Tang and N. Jiao, J. Am. Chem. Soc., 2012, 134, 18924 CrossRef CAS PubMed.
- For selected reviews on isocyanide insertions, see: (a) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (b) T. Vlaar, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2013, 52, 7084 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05670a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |