Open Access Article
Open Access ArticleHeterogeneous ozonolysis of alkyl substituted-polycyclic aromatic hydrocarbons (AlkPAHs) in the atmosphere†
Vera
Zaherddine
 ab,
Elisabeth
Galarneau
ab and
Arthur W. H.
Chan
ab,
Elisabeth
Galarneau
ab and
Arthur W. H.
Chan
 *a
*a
aDepartment of Chemical Engineering and Applied Chemistry, University of Toronto, Canada. E-mail: arthurwh.chan@utoronto.ca
bAir Quality Research Division, Science and Technology Branch, Environment and Climate Change Canada, Canada
First published on 25th April 2024
Abstract
Polycyclic aromatic compounds (PACs) encompass a range of organic pollutants, including polycyclic aromatic hydrocarbons (PAHs), alkyl-substituted PAHs (AlkPAHs), and others. PAHs have been extensively studied due to their environmental and health implications. AlkPAHs, however, have received relatively less attention, despite recent evidence suggesting their greater abundances in ambient air. Given their prevalence and potential risks, investigating the atmospheric transformation of AlkPAHs is crucial. This work focuses on the heterogeneous oxidation of AlkPAHs, specifically addressing the influence of alkyl groups on reaction kinetics. Oxidation by gas phase ozone was conducted on quartz filters, which serve as models for silica surfaces on which PACs can deposit with minimal chemical interactions. The results reveal that AlkPAHs react faster with ozone than PAHs do, with reaction rates increasing with higher alkyl group substitutions. Furthermore, oxygenated polycyclic aromatic hydrocarbons (OPAHs) were formed during the oxidation of 1-methylpyrene, with greater diversity than those from pyrene. These products are more polar and potentially more toxic than parent compounds. In conclusion, this research advances our understanding of PAC oxidation, focusing on AlkPAHs' heterogeneous oxidation, the influence of alkyl groups, and the formation of OPAHs. These insights have significant implications for air quality, health risk assessments, and the fate of PACs in the environment.
Environmental significancePolycyclic aromatic compounds are emitted into the atmosphere from human activities. In the atmosphere, polycyclic aromatic hydrocarbons (PAHs) transform into potentially toxic compounds. Recent studies, however, have found that alkylated polycyclic aromatic hydrocarbons (Alk-PAHs) are far more abundant. Knowledge of Alk-PAH reactions will lead to better estimation of their fate and impacts. This study reports on the laboratory investigation of Alk-PAHs on surface mimics of atmospheric particles. Alk-PAHs react significantly faster than PAHs, and transform into a larger variety of products. Their lifetimes on these surfaces are on the order of days. The results of this study suggest that these reactions occur within urban areas where they are emitted, and should therefore be considered when assessing inhalation risks. |
Introduction
Polycyclic Aromatic Compounds (PACs) are a diverse family of organic pollutants that include unsubstituted Polycyclic Aromatic Hydrocarbons (PAHs), alkyl-substituted PAHs (AlkPAHs), nitrogen-containing PAHs, oxygen-containing PAHs (OPAHs), and sulfur-containing PAHs. These compounds are emitted from various sources, both natural and anthropogenic, such as traffic emissions, wood combustion, and petroleum products.1–4 PAHs have been investigated in detail due to their recognized environmental and health implications.4–10 In Canada, for example, PAHs are listed as toxic substances under the Canadian Environmental Protection Act, and several PAHs are classified as Group 1 carcinogens by the International Agency for Research on Cancer, signifying their carcinogenic potential.11,12 In contrast to the significant attention given to PAHs, AlkPAHs have not been studied extensively, even though studies have shown that AlkPAHs may possess greater toxicity than PAHs.13,14 Notably, recent research has reported higher AlkPAH levels than PAH levels at several Canadian locations.15–18Given the growing evidence of AlkPAHs' prevalence in the atmosphere and their potential health risks, investigating their transformations in the atmosphere is imperative. PACs undergo photolysis upon ultraviolet exposure and oxidation through reactions with gas-phase oxidants.2,19–23 Depending on the oxidants in question, these oxidation processes lead to the formation of secondary OPAHs and NPAHs, which are often more toxic than the parent PAHs.24–27 These oxidation processes can be classified into homogeneous, occurring in the gas phase, or heterogeneous reactions, taking place on the surfaces of particles in the atmosphere.2,22,23 While homogeneous oxidation of PAHs has been extensively investigated, the research on heterogeneous oxidation of AlkPAHs remains limited, despite its potential significance.1,5–10
Here we hypothesize that alkyl groups, acting as electron-donating groups, stabilize reaction intermediates and increase reaction rates.5,28 To test this hypothesis, the research aims to elucidate the impact of alkyl groups on PAC reactions and to assess the influence of various alkyl groups on the reaction rates. Gas phase AlkPAHs have been shown to react faster with higher alkyl group substitutions, but the relative rates of heterogeneous oxidation, which are more important sinks for PACs with higher molecular weights, are unknown.5
Additionally, the formation of OPAHs from the oxidation of AlkPAHs remains relatively unexplored, while their potential health risks are significant. One study reported a discrepancy between rate of PAHs decay and OPAHs formation which suggests the important role of AlkPAH oxidation.10 Therefore, identifying the products and understanding the mechanisms of PAC oxidation is crucial, as it offers insights into the types of OPAHs formed and can inform assessments of toxicity. Two proposed mechanisms for the ozonolysis of PAHs, involving ring-opening mechanisms and oxygen addition without ring opening, result in OPAHs with different functional groups.29 Identifying the prevailing mechanisms can improve our understanding of the specific OPAHs formed and their potential health effects. Furthermore, if AlkPAHs are found to react more rapidly with ozone than PAHs, they could play a more substantial role in secondary OPAH formation in the atmosphere.
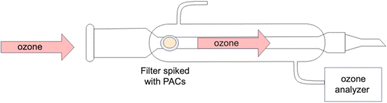
Heterogeneous oxidation introduces complexities not encountered in homogeneous oxidation, primarily due to interactions with various surfaces. Studies indicate that the nature of the surface on which PACs are deposited plays a crucial role in determining their reaction rates. Additionally, the presence of other compounds in the atmosphere can influence the reactions of PACs on surfaces. To gain a more fundamental understanding of the impact of alkyl groups on the reaction kinetics, a simplified system using quartz filters is used here. By depositing PACs onto these filters and exposing them to ozone in a controlled flow reactor under varying conditions, we assess the decay of PACs over time and in response to varying ozone concentrations, and characterize the oxidation products formed from these reactions.
Materials and methods
Sample preparation and analysis for kinetics
Hexane (HEX, ACS) and acetone (ACT, HPLC grade) were purchased from Fisher Chemicals. Dichloromethane (DCM, reagent grade) were purchased from Caledon Laboratory Chemicals. Standard PAHs mix and internal standards were purchased from AccuStandard. Pyrene (PYR, 98% purity), pyrene-d12 (PYR-d12, 98% purity) and 1-methylpyrene (1-MP, 98% purity) were purchased from Millipore Sigma. Chrysene (CHRY, 98% purity), 6-methylchrysene (6-MC, 98% purity) and 6-ethylchrysene (6-EC, 98% purity) were purchased from AccuSandard. A mixture of deuterated PAHs containing naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12 and perylene-d12 were purchased from AccuStandard. Quartz filters (47 mm diameter) were purchased from Whatman (grade QMA).Each quartz filter was cut into 4 equal pieces and baked at 500 °C overnight. Each of the compounds, including pyrene, chrysene, 1-methylpyrene, 6-methylchrysene, and 6-ethylchrysene, was dissolved in DCM and spiked onto a filter. DCM was evaporated after 90–100 s at room temperature leaving a layer of ∼1.6 μg of PAC on the filter. Based on the surface area of the filter, we expect the average surface coverage of the PAC film to be ≪ 1 molecule per nm2, so the film formed on the filter is expected to be a monolayer. The filter was then placed in a glass flow tube (49 cm in length, 3 cm internal diameter) and exposed to ozone (Fig. 1). Ozone was generated by passing ∼99% pure oxygen through a ozone generator (UVP) at a 1 L min−1 flowrate. Ozone levels were monitored using an ozone analyzer (Thermo 49i). Experiments were done at room temperature. PACs were exposed to ∼1 ppm, 0.8 ppb, 0.6 ppb and 0.3 ppb of ozone for 0–5 h, and the loss of PACs was reported for these time intervals. For each ozone concentration and exposure time, triplicate experiments on different filters were performed.
After exposure to ozone, the filters were spiked with a known amount of pyrene-d12 and extracted in 15 mL of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane/acetone mixture by sonication for 30 min. Extracted samples were blown down to 1 mL using a nitrogen evaporator (N-EVAP, Organomation) and then spiked with a known amount of deuterated PAH mixture. The PACs were analyzed using Thermo Scientific Trace 1310 gas chromatography mass spectrometer (GC/MS) with an Rxi-5ms (30 m × 0.25 mm ID × 0.25 μm film thickness) column. For each sample, 2.0 μL of the blown down extract was injected into the GC via an autosampler. The operation mode was splitless at 344 kPa pressure and 320 °C inlet temperature. Purge flow is turned on directly after injection at a rate of 50 mL min−1. The column temperature started at 80 °C with 1 min hold time, increased up to 200 °C at a rate of 25 °C min−1 then increased to 335 at 8 °C min−1 with 6 min hold time. The carrier gas was helium (He) at a constant flow rate of 0.9 mL min−1. The mass spectrometer was operated with electron impact ionization in full scan mode.
1 hexane/acetone mixture by sonication for 30 min. Extracted samples were blown down to 1 mL using a nitrogen evaporator (N-EVAP, Organomation) and then spiked with a known amount of deuterated PAH mixture. The PACs were analyzed using Thermo Scientific Trace 1310 gas chromatography mass spectrometer (GC/MS) with an Rxi-5ms (30 m × 0.25 mm ID × 0.25 μm film thickness) column. For each sample, 2.0 μL of the blown down extract was injected into the GC via an autosampler. The operation mode was splitless at 344 kPa pressure and 320 °C inlet temperature. Purge flow is turned on directly after injection at a rate of 50 mL min−1. The column temperature started at 80 °C with 1 min hold time, increased up to 200 °C at a rate of 25 °C min−1 then increased to 335 at 8 °C min−1 with 6 min hold time. The carrier gas was helium (He) at a constant flow rate of 0.9 mL min−1. The mass spectrometer was operated with electron impact ionization in full scan mode.
Pyrene-d12 was used to account for extraction loss and phenanthrene-d10 in the deuterated mixture was used to account for evaporation losses during volume blowdown. Eqn (1) was used to correct PAC concentration measured by GC/MS using the deuterated standards added.
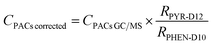 | (1) |
 | (2) |
Triplicate samples of PACs deposited on filters with no ozone exposure were also extracted to calculate the recovery of analytes after extraction. The recovery of the pyrene, 1-methylpyrene, chrysene, 6-methylchrysene and 6-ethylchrysene are 93%, 98%, 96%, 89% and 89%, respectively, with an analytical instrument error of 10%. The analytical instrument error was calculated from the standard deviation of triplicate runs of the same known concentration of compounds. To examine contamination by background PAHs, a blank filter was extracted using the same procedure as the samples to account for any background PAHs not removed by pre-baking the filters. No PACs appeared on the blank filter and no blank correction was required.
In addition to ozonolysis experiments, evaporation loss of PACs on filters was also assessed by exposing filters with pyrene to clean compressed air for the same time intervals, and the loss of pyrene with time was monitored. The evaporation loss of pyrene from the filter over 5 h was less than 10%, which is within the 10% analytical error. Since pyrene is the most volatile compound out of the tested PACs, the evaporation loss for all compounds studied is considered negligible.
Pseudo-first and second order rate constant
The pseudo-first order rate constant was calculated from the degradation of PACs with time. Here we model the kinetics of PAC oxidation with this general equation:7,9,19,21–23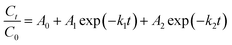 | (3) |
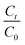 is the decay of the PAC, A0 is the residual fraction, A1, A2 are the fractions of PAC decaying with two different timescales, and k1 and k2 are the associated pseudo-first order rate constants (s−1). In some cases, PAC decay with a single timescale, and the time trend can be simplified into a single decay first order decay term (A1 and k1), as observed by Perraudin et al. (2007)9 for some PAHs, which reacted to completion with a first order exponential decay. In other cases, PAHs did not react completely, and A0 is non-zero to capture the trend.9 In another study done by Zhou et al. (2019),30 a double exponential decay rate was reported for the oxidation of benzo[a]pyrene where A0 would be zero and the two decay rates would be non-zero. Based on these observations from the literature and from our study, eqn (3) is used as a generalized equation of the heterogeneous oxidation of PACs.
is the decay of the PAC, A0 is the residual fraction, A1, A2 are the fractions of PAC decaying with two different timescales, and k1 and k2 are the associated pseudo-first order rate constants (s−1). In some cases, PAC decay with a single timescale, and the time trend can be simplified into a single decay first order decay term (A1 and k1), as observed by Perraudin et al. (2007)9 for some PAHs, which reacted to completion with a first order exponential decay. In other cases, PAHs did not react completely, and A0 is non-zero to capture the trend.9 In another study done by Zhou et al. (2019),30 a double exponential decay rate was reported for the oxidation of benzo[a]pyrene where A0 would be zero and the two decay rates would be non-zero. Based on these observations from the literature and from our study, eqn (3) is used as a generalized equation of the heterogeneous oxidation of PACs.
The heterogeneous oxidation of PACs with ozone is a bimolecular reaction which is dependent on ozone concentration7,19,22 and often follows a second order rate law with ozone concentration. Assuming that ozone is in large excess, the second order rate constant was obtained by regressing the pseudo-first order rate constant against the ozone concentration. When two terms from eqn (3) were needed to capture the time trend, the faster rate constant was used to obtain the second order rate constant.
Oxidation products
A semi-quantitative analysis of OPAH products formed from ozonolysis was conducted. A set of triplicate filters was prepared, representing exposure times of 0 minutes and 300 minutes to 1 ppm of ozone for pyrene and 1-methylpyrene. The extraction of compounds from these filters followed the method previously described. Subsequently, the extracted samples were evaporated to dryness. The dried samples were then reconstituted in a 50/50 acetonitrile/water solution and loaded into glass autosampler vials for LC/MS analysis.The analysis was conducted using a Q-Exactive liquid chromatography-mass spectrometry (LC/MS) system equipped with electrospray ionization (ESI) and a quadrupole-orbitrap mass spectrometer (Thermo). Chromatographic separation was achieved with a Waters BEH C18 column (50 × 2.1 mm, 1.7 μm) equipped with a guard column, maintained at a column temperature of 50 °C. An autosampler, set at 10 °C, injected a fixed volume of 10 μL into the system. Elution of compounds was performed using a gradient elution method with two eluents: eluent A, consisting of 20 mM ammonium acetate and 20 mM ammonium hydroxide in H2O, and eluent B, composed of acetonitrile. The elution was carried out at a flow rate of 0.4 mL min−1.
The mass spectrometry analysis included a full MS scan, covering a mass-to-charge ratio (m/z) range from 100 to 1000. This full scan operated at a resolution of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, with an automatic gain control (AGC) target set at 3 × 106. Furthermore, the analysis involved polarity switching between positive and negative modes to ensure comprehensive compound detection. Data-dependent MS2 scans were conducted at a resolution of 17
000, with an automatic gain control (AGC) target set at 3 × 106. Furthermore, the analysis involved polarity switching between positive and negative modes to ensure comprehensive compound detection. Data-dependent MS2 scans were conducted at a resolution of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, an AGC target of 1 × 105, and an isolation window of 0.4 m/z. Results were filtered systematically and details are further explained in ESI.†
500, an AGC target of 1 × 105, and an isolation window of 0.4 m/z. Results were filtered systematically and details are further explained in ESI.†
Results and discussion
Heterogeneous oxidation
 | ||
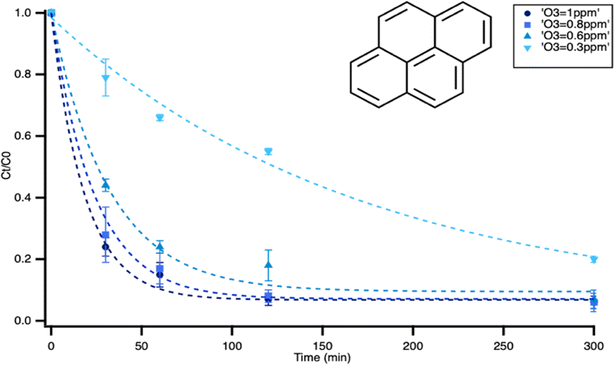
| Fig. 3 Structure and evolution of 1-methylpyrene concentration with exposure time to 1, 0.8, 0.6 and 0.3 ppm of ozone with double exponential fit. | ||
After 300 minutes of ozone exposure, both pyrene and 1-methylpyrene had reacted to completion. Pyrene exhibited a first-order exponential decay at all ozone concentrations, showing an increasing oxidation rate with higher ozone concentrations. 1-Methylpyrene displayed a notably faster reaction rate than pyrene. As shown in Fig. 3, 1-methylpyrene showed a faster initial decay within the first 10 minutes, followed by a slower rate, indicative of a double exponential decay pattern. This two-rate behavior has been observed previously and was attributed to crust formation, as proposed by Zhou et al. (2019).30 During oxidation, products accumulate and form a layer on the surface of certain PACs, hindering the sorption of ozone to the surface and thereby slowing down the reaction. In this case, pyrene exhibited a slower reaction, resulting in a delayed formation of products that may account for the absence of the two-rate behavior. This rate behavior is inconsistent with our monolayer assumption based on calculated surface coverage. However, this inconsistency was also reported in the study by Zhou et al. (2019).30 It was suggested that PAHs may cluster, and form stacked layers even if there is enough surface area to have a monolayered distribution.30 Without detailed knowledge of PAC microstructure on these surfaces, we will focus our analysis on the rate behavior in the rapid first decay, which represent kinetics without mass transport limitations.
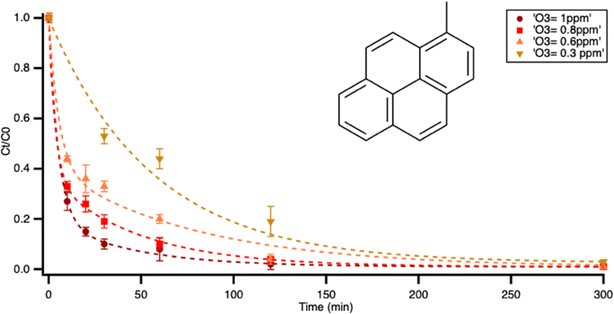
From Fig. 4, the second-order rate constants for pyrene and 1-methylpyrene were calculated as (4.3 ± 0.2) × 10−17 and (1.8 ± 0.2) × 10−16 cm3 per molecules, respectively. In other words, the ozonolysis rate constant of 1-methylpyrene is about 4 times higher than that of pyrene. In the experiments, the relationship between the first-order rate constant and ozone concentration remained within the linear range, suggesting that the heterogeneous reaction was ozone-limited. In this regime, an increase in bulk gas-phase ozone concentration led to a greater amount of ozone available at the surface for the reactions with pyrene and 1-methylpyrene.
 | ||
| Fig. 4 Pseudo-first order rate constants of reaction of pyrene and the fast phase rate of 1-methylpyrene with ozone as a function of ozone concentration. | ||
We also examined the heterogeneous ozonolysis of chrysene, 6-methylchrysene, and 6-ethylchrysene, chosen for their commercial availability and alkyl substitutions at the same carbon position, varying from methyl to ethyl groups. Notably, chrysene did not decay completely by the end of the experiment, consistent with its lower reactivity as observed in previous studies.9Fig. 5 illustrates that, at all ozone concentrations, chrysene displayed a delay period of 30 minutes before commencing decay. This complex trend currently lacks a known mechanistic explanation and should be a topic of future investigation.
 | ||
| Fig. 5 Structure and evolution of chrysene concentration with exposure time to 1, 0.8, 0.6 and 0.3 ppm of ozone with exponential fit. | ||
To investigate the effect of methyl addition, we compare ozonolysis rates between pyrene and 1-methylpyrene, as well as between chrysene and 6-methylchrysene. Additionally, the effect of different alkyl group substitutions on heterogeneous oxidation was assessed by exposing 6-ethylchrysene to the same conditions as chrysene and 6-methylchrysene, serving as a basis for comparison (refer to Fig. S1 and S2†). The calculated second-order rate constants for chrysene and 6-methylchrysene, and 6-ethylchrysene were (6.9 ± 0.6) × 10−18, (1.0 ± 0.1) × 10−17, and (1.3 ± 0.1) × 10−17 cm3 per molecules, respectively, as shown in Fig. 6.
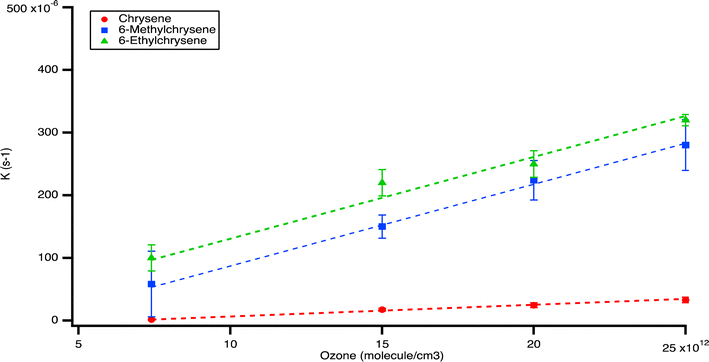 | ||
| Fig. 6 Pseudo-first order rate constants of reaction of chrysene, 6-methylchrysene and 6-ethylchrysene with ozone as a function of ozone concentration. | ||
These results, in conjunction with the findings from the pyrene experiments, suggest that the methylation of PAHs enhances their reaction rate. Methyl groups, acting as electron-donating groups, may accelerate the reaction by stabilizing intermediates. Furthermore, the results from chrysene, 6-methylchrysene, and 6-ethylchrysene confirm that an increase in the number of carbon atoms in the substituent alkyl groups also increases the reaction rate. This observation aligns with trends observed in homogeneous oxidation,5 where AlkPAHs reacted faster than PAHs, and the rate increased with greater substitution. Unfortunately, due to limitations in commercial availability, PACs with multiple alkyl groups could not be examined in this study. For homogeneous oxidation, their reaction rates were reported to be even higher.5 Our findings underscore the significance of alkyl group substitutions in influencing the heterogeneous oxidation of PAHs.
| PACs | Second order rate constant (cm3 per molecules) | Lifetime at 30 ppb ozone concentration (h) | Lifetime at 30 ppb ozone concentration (day) |
|---|---|---|---|
| Pyrene | (4.3 ± 0.2) × 10−17 | 53 | 2.2 |
| 1-Methylpyrene | (1.8 ± 0.2) × 10−16 | 13 | 0.5 |
| Chrysene | (6.9 ± 0.6) × 10−18 | 329 | 13.7 |
| 6-Methylchrysene | (1.0 ± 0.1) × 10−17 | 227 | 9.4 |
| 6-Ethylchrysene | (1.3 ± 0.1) × 10−17 | 174 | 7.3 |
Heterogeneous oxidation of PACs with ozone is an important reaction, but other oxidants, such as OH and NO2, are also important. In the ambient atmosphere, PAHs can remain in the atmosphere for weeks, and there may be other reactions and interactions not represented in this experiment. For example, graphite surfaces stabilize PACs towards oxidation due to the strong interactions.31 Also, the presence of other pollutants with PACs can hinder the oxidation.23 The purpose of this study is to take a first look at the effect of alkylation on reaction rates in a simple model. Further studies should be conducted to get a deeper understanding of AlkPAHs and their fate in the atmosphere when associated with particulate matter of different composition.
Nevertheless, the faster oxidation of AlkPAHs is notable. For less reactive species, such as chrysene, the addition of methyl groups reduces ozonolysis timescales from weeks to a few days, allowing the heterogeneous reactions to be a notable pathway for loss of PACs. Heterogeneous ozonolysis of the most reactive AlkPAHs, such as 1-methylpyrene, can occur on timescales of less than 1 day. These timescales are smaller than those for regional atmospheric transport, which suggests these reactions are important considerations for local air quality. The reaction of PACs with ozone leads to the production of OPAHs, which are more polar than PACs, increasing their potential ability to cause oxidative stress and subsequent negative impacts on human health.3,24–27 From our results, formation timescales of OPAH from AlkPAHs can be on the order of <1 day to days (compared to days to weeks from PAHs). These timescales suggest more localized OPAH formation in and around major sources, which may also be major population centres. As mentioned earlier, observed concentrations of AlkPAHs in urban and industrial regions in Canada exceed those of PAHs. Together these two factors make oxidation of AlkPAHs an important source of OPAHs in urban areas. Products of PAHs oxidation have been identified in previous studies, while much less is known about the products of AlkPAHs oxidation.6,10,32 Given the importance of AlkPAHs to OPAH production, there is a need to identify their oxidation products and understand their formation.
Oxidation products
The products from ozonolysis of pyrene and 1-methylpyrene were identified in this study. Following the systematic filtering of the oxidation product analysis explained in ESI,† a total of 21 chemical formulae ranging from C13 to C17 are reported. Table 2 shows the chemical formulae, precursor and number of isomers for 21 chemical formulas. Here we focus on C15–C17 products to investigate the potential mechanisms leading to their formation.| Precursor | Chemical formula | Molecular weight | Number of isomers |
|---|---|---|---|
| Pyrene | C15 H8 O | 204.0573 | 3 |
| C15 H10 O | 206.0730 | 1 | |
| C16 H8 O2 | 232.0520 | 3 | |
| C16 H10 O2 | 234.0677 | 3 | |
| C16 H10 O3 | 250.0627 | 1 | |
| 1-Methylpyrene | C13 H8 O2 | 196.0522 | 1 |
| C15 H10 O2 | 222.0677 | 1 | |
| C15 H10 O3 | 238.0626 | 1 | |
| C16 H10 O | 218.0729 | 2 | |
| C16 H12 O | 220.0886 | 2 | |
| C16 H12 O2 | 236.0835 | 1 | |
| C17 H10 O2 | 246.0679 | 2 | |
| C17 H12 O2 | 248.0833 | 3 | |
| C17 H10 O3 | 262.0625 | 3 | |
| C17 H12 O3 | 264.0782 | 1 | |
| C17 H12 O5 | 296.0686 | 1 | |
| C17 H12 O6 | 312.0639 | 1 | |
| Pyrene and 1-methylpyrene | C14 H8 O2 | 208.0522 | 1 |
| C16 H8 O3 | 248.0470 | 2 | |
| C16 H10 O4 | 266.0576 | 1 | |
| C16 H10 O5 | 282.0529 | 1 |
Here the discussion of products arising from the oxidation of pyrene was informed by previous studies,6,10,29,32 which provided valuable insights into the potential reaction pathways. The studies presented a mechanism comprising two distinct routes: (1) a ring-retaining pathway and (2) a ring-opening pathway.6,10,29,32 Notably, molecules with molecular weights of 206, 208, 232, 234, 248, 250, and 266 were identified both in the present study and in these cited works. Here, the exact masses were quantified, which allows for identification of elemental composition. These elemental formulae are consistent with molecular structures proposed in the literature. 1-Hydroxypyrene (MW 218) was not observed among the pyrene oxidation products in this study which may be due to low amount produced or our filtering criteria.
Previous studies have examined pyrene oxidation and the OPAHs formed.6,10,29,32 On the other hand, 1-methylpyrene has not been investigated yet. In this study, the products of OPAHs were identified for pyrene and 1-methylpyrene. Three oxidation products with chemical formula C16H8O3, C16H10O4 and C16H10O5 were common between pyrene and 1-methylpyrene. The identical elemental formulae suggest that one of the pathways in the oxidation of 1-methylpyrene may involve a demethylation step, potentially during ring opening. A previous study examined heterogeneous oxidation of ambient particles on a filter10 and found that the amount of OPAHs formed was greater than what the measured PAHs can account for. It was hypothesized that alkPAHs contribute to OPAH formation,10 and the overlap in oxidation products between pyrene and 1-methylpyrene is consistent with this hypothesis.
Other than the 3 products in common with pyrene, 1-methylpyrene also produced other compounds not identified from pyrene oxidation. A total of 11 products were identified as products of 1-methylpyrene oxidation only. 5 out of 11 can be considered alkylated equivalents to the non-alkylated pyrene products with a molecular mass difference of 14.0156 (that of –CH2–) presented in Table 3. The prevalence of alkylated analogs suggests that 1-methylpyrene follow similar oxidation pathways to that of pyrene, which lead to alkylated pyrene products. The 6 remaining OPAHs produced from 1-methylpyrene are unique to 1-methylpyrene with no apparent relation with pyrene products. The addition of the methyl group to the aromatic rings may lead to other pathways which would produce a larger variety OPAHs than pyrene.
| Pyrene product | Molecular weight | Molecular weight + [CH2] | Analogous 1-methylpyrene product |
|---|---|---|---|
| C15 H8 O | 204.0573 | 218.0729 | C16 H10 O |
| C15 H10 O | 206.0730 | 220.0886 | C16 H12 O |
| C16 H8 O2 | 232.0520 | 246.0679 | C17 H10 O2 |
| C16 H10 O2 | 234.0677 | 248.0833 | C17 H12 O2 |
| C16 H10 O3 | 250.0627 | 264.0782 | C17 H12 O3 |
Based on the oxidation products identified in this study, 1-methylpyrene produced a larger variety of OPAHs than pyrene. Owing to the lack of authentic standards, the abundance cannot be quantified in this study. However, based on previous work by Ringuet et al. (2012),10 there is reason to believe that alkylated PAHs are a major source of OPAHs. Some of the OPAHs have not been identified before and none of the OPAHs are routinely monitored. OPAHs are more toxic than parent PAHs24–27 suggesting that the lack of identification and monitoring of OPAHs may lead to underestimation of air toxicity. In addition, 1-methylpyrene produced some pyrene products which suggests that 1-methylpyrene contributes to the formation of those products in the atmosphere. As shown from the kinetic study, 1-methylpyrene reacts faster than pyrene, which will result in faster production of those compounds.
1-ethylpyrene is only one of the alkylated analogs of pyrene; other AlkPAHs like 4,5-dimethylpyrene and 1-propylpyrene have been reported in the atmosphere.15,17,18 It is worth noting that the levels of AlkPAHs in the atmosphere surpass those of the original PAHs, and each PAH can have multiple alkylated analogs.15,17,18 Based on the findings of this study, it is evident that AlkPAHs exhibit a higher reactivity with ozone compared to PAHs and produce a greater variety of OPAHs. This greater reactivity and increased OPAH production by AlkPAHs introduce a previously undiscovered dimension to our understanding of PACs. Additionally, it is important to explore and comprehend the mechanism of oxidation, along with factors such as relative humidity. In light of the health implications associated with AlkPAHs and OPAHs, it is imperative to conduct in-depth monitoring and research on these compounds to better understand their effect on the atmosphere and human health.
Conclusions
In this study we report on heterogeneous oxidation of AlkPAHs, and the formation of OPAHs. The investigation demonstrated that AlkPAHs react faster with ozone than PAHs do, by a factor of 2 to 4. Furthermore, higher alkyl substitution leads to faster reactions. This trend in reaction kinetic is likely due to the electron-donating nature of alkyl groups which stabilizes intermediates. Furthermore, our results show that 1-methylpyrene produces more oxidation products than pyrene. Some of the products are identical to those from pyrene, others are alkylated analogs of pyrene products, and the rest are 1-methylpyrene products do not belong in either category. The identification of these oxidation products suggests that while there may be common and analogous reaction mechanisms between PAH and AlkPAH oxidation, there may also be other reactions that are currently unknown. Given the higher toxicity of OPAHs compared to PACs and their potential adverse health effects, there is a need for more extensive research to identify and quantify the oxidation products of AlkPAHs.It should be noted that the current results were obtained from PACs deposited on silica surfaces, representing the upper bound of reaction kinetics. Further research should encompass a broader range of atmospheric surfaces, such as graphite or organic surfaces, to better represent PAC oxidation on atmospheric particles. We were also limited to commercially available isomers. As a result, we were unable to study AlkPAHs that are multiply substituted. More research is warranted to understand the heterogeneous oxidation of various AlkPAHs.
The findings from this study contribute to the identification of OPAHs and an enhanced understanding of the pathways governing their formation. In summary, this study provides insights into oxidation reactions of PACs, particularly concerning AlkPAHs and the impact of alkyl groups, as well as the formation of OPAHs. Given that our study suggests oxidation timescales are less than or similar to regional transport, the potential for long range transport of AlkPAHs is less than that for PAHs, but the exposure to secondary OPAHs near emission sources of their precursors may be more significant. Coupled with the potentially greater toxicity of OPAHs shown by previous studies, these results have important implications for assessing health risks, and understanding the fate of PACs in the environment.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
This work was funded by Environment and Climate Change Canada (ECCC).References
- T. D. Behymer and R. A. Hites, Photolysis of polycyclic aromatic hydrocarbons adsorbed on fly ash, Environ. Sci. Technol., 1988, 22(11), 1311–1319, DOI:10.1021/es00176a011.
- B. J. Finlayson-Pitts and J. N. Pitts, Chemistry of the Upper and Lower Atmosphere, San Diego, Academic Press, 2000 Search PubMed.
- T. Letzel, E. Rosenberg, R. Wissiack, M. Grasserbauer and R. Niessner, Separation and identification of polar degradation products of benzo[a]pyrene with ozone by atmospheric pressure chemical ionization–mass spectrometry after optimized column chromatographic clean-up, J. Chromatogr. A, 1999, 855(2), 501–514, DOI:10.1016/s0021-9673(99)00716-5.
- J. Yao, Z. Huang and S. J. Masten, The ozonation of pyrene: Pathway and product identification, Water Res., 1998, 32(10), 3001–3012, DOI:10.1016/s0043-1354(98)00056-6.
- R. Atkinson and J. Arey, Mechanisms of the Gas-Phase Reactions of Aromatic Hydrocarbons and PAHS with OH and NO3 Radicals, Polycycl. Aromat. Compd., 2007, 27(1), 15–40, DOI:10.1080/10406630601134243.
- R. E. Cochran, H. Jeong, S. Fisseha Derseh, A. Gowan, J. Beránek and A. Kubátová, Identification of products formed during the heterogeneous nitration and ozonation of polycyclic aromatic hydrocarbons, Atmos. Environ., 2016, 128, 92–103, DOI:10.1016/j.atmosenv.2015.12.036.
- T. Kahan, N. Kwamena and D. Donaldson, Heterogeneous ozonation kinetics of polycyclic aromatic hydrocarbons on organic films, Atmos. Environ., 2006, 40(19), 3448–3459, DOI:10.1016/j.atmosenv.2006.02.004.
- N. A. Kwamena, M. E. Earp, C. J. Young and J. P. Abbatt, Kinetic and Product Yield Study of the Heterogeneous Gas−Surface Reaction of Anthracene and Ozone, J. Phys. Chem. A, 2006, 110(10), 3638–3646, DOI:10.1021/jp056125d.
- E. Perraudin, H. Budzinski and E. Villenave, Kinetic Study of the Reactions of Ozone with Polycyclic Aromatic Hydrocarbons Adsorbed on Atmospheric Model Particles, J. Atmos. Chem., 2007, 56(1), 57–82, DOI:10.1007/s10874-006-9042-x.
- J. Ringuet, A. Albinet, E. Leoz-Garziandia, H. Budzinski and E. Villenave, Reactivity of polycyclic aromatic compounds (PAHs, NPAHs and OPAHs) adsorbed on natural aerosol particles exposed to atmospheric oxidants, Atmos. Environ., 2012, 61, 15–22, DOI:10.1016/j.atmosenv.2012.07.025.
- Canadian Environmental Protection Act, Declaration of Environmental Rights and Responsibilities, 1999, S.C. 1999, c. 33, s. 5(1) Search PubMed.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures, IARC Monogr. Eval. Carcinog. Risks Hum., 2010, 92, 1–853 Search PubMed.
- S. B. Hawthorne, D. J. Miller and J. P. Kreitinger, Measurement of total polycyclic aromatic hydrocarbon concentrations in sediments and toxic units used for estimating risk to benthic invertebrates at manufactured gas plant sites, Environ. Toxicol. Chem., 2006, 25(1), 287, DOI:10.1897/05-111r.1.
- D. H. Pcchillips, P. L. Grover and P. Sims, A quantitative determination of the covalent binding of a series of polycylic hydrocarbons to DNA in mouse skin, Int. J. Cancer, 1979, 23(2), 201–208, DOI:10.1002/ijc.2910230211.
- M. Moradi, H. Hung, J. Li, R. Park, C. Shin, N. Alexandrou, M. A. Iqbal, M. Takhar, A. W. H. Chan and J. R. Brook, Assessment of Alkylated and Unsubstituted Polycyclic Aromatic Hydrocarbons in Air in Urban and Semi-Urban Areas in Toronto, Canada, Environ. Sci. Technol., 2022, 56(5), 2959–2967, DOI:10.1021/acs.est.1c04299.
- J. K. Schuster, T. Harner, K. Su, A. Eng, A. Wnorowski and J. Charland, Temporal and Spatial Trends of Polycyclic Aromatic Compounds in Air across the Athabasca Oil Sands Region Reflect Inputs from Open Pit Mining and Forest Fires, Environ. Sci. Technol. Lett., 2019, 6(3), 178–183, DOI:10.1021/acs.estlett.9b00010.
- A. Wnorowski, Y. Aklilu, T. Harner, J. Schuster and J. Charland, Polycyclic aromatic compounds in ambient air in the surface minable area of Athabasca oil sands in Alberta (Canada), Atmos. Environ., 2021, 244, 117897, DOI:10.1016/j.atmosenv.2020.117897.
- A. Wnorowski, D. Harnish, Y. Jiang, V. Celo, E. Dabek-Zlotorzynska and J. Charland, Assessment and Characterization of Alkylated PAHs in Selected Sites across Canada, Atmosphere, 2022, 13(8), 1320, DOI:10.3390/atmos13081320.
- M. Cazaunau, K. Le Ménach, H. Budzinski and E. Villenave, Atmospheric Heterogeneous Reactions of Benzo(a)pyrene, Z. Phys. Chem., 2010, 224(7–8), 1151–1170, DOI:10.1524/zpch.2010.6145.
- E. Galarneau, Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment, Atmos. Environ., 2008, 42(35), 8139–8149, DOI:10.1016/j.atmosenv.2008.07.025.
- K. Miet, K. Le Menach, P. Flaud, H. Budzinski and E. Villenave, Heterogeneous reactions of ozone with pyrene, 1-hydroxypyrene and 1-nitropyrene adsorbed on particles, Atmos. Environ., 2009, 43(24), 3699–3707, DOI:10.1016/j.atmosenv.2009.04.032.
- N. A. Kwamena, M. G. Staikova, D. J. Donaldson, I. J. George and J. P. Abbatt, Role of the Aerosol Substrate in the Heterogeneous Ozonation Reactions of Surface-Bound PAHs, J. Phys. Chem. A, 2007, 111(43), 11050–11058, DOI:10.1021/jp075300i.
- S. Zhou, A. K. Lee, R. D. McWhinney and J. P. Abbatt, Burial Effects of Organic Coatings on the Heterogeneous Reactivity of Particle-Borne Benzo[a]pyrene (BaP) toward Ozone, J. Phys. Chem. A, 2012, 116(26), 7050–7056, DOI:10.1021/jp3030705.
- J. Koenig, E. Balfanz, W. Funcke and T. Romanowski, Determination of oxygenated polycyclic aromatic hydrocarbons in airborne particulate matter by capillary gas chromatography and gas chromatography/mass spectrometry, Anal. Chem., 1983, 55(4), 599–603, DOI:10.1021/ac00255a004.
- D. U. Pedersen, J. L. Durant, B. W. Penman, C. L. Crespi, H. F. Hemond, A. L. Lafleur and G. R. Cass, Human-Cell Mutagens in Respirable Airborne Particles in the Northeastern United States. 1. Mutagenicity of Fractionated Samples, Environ. Sci. Technol., 2003, 38(3), 682–689, DOI:10.1021/es0347282.
- J. N. Pitts, H. Paur, B. Zielinska, J. Arey, A. M. Winer, T. Ramdahl and V. Mejia, Factors influencing the reactivity of polycyclic aromatic hydrocarbons adsorbed on filters and ambient POM with ozone, Chemosphere, 1986, 15(6), 675–685, DOI:10.1016/0045-6535(86)90033-0.
- D. Yaffe, Y. Cohen, J. Arey and A. J. Grosovsky, Multimedia Analysis of PAHs and Nitro-PAH Daughter Products in the Los Angeles Basin, Risk Anal, 2001, 21(2), 275–294, DOI:10.1111/0272-4332.212111.
- J. Clayden, N. Greeves and S. Warren, Organic Chemistry, Oxford University Press, 2001 Search PubMed.
- X. Qi, X. Pang, Y. Hong, Y. Wang, S. Lou, J. Feng, P. Cheng and Z. Zhou, Real-time analysis of the homogeneous and heterogeneous reactions of pyrene with ozone by SPAMS and CRD-EAS, Chemosphere, 2019, 234, 608–617, DOI:10.1016/j.chemosphere.2019.06.050.
- S. Zhou, B. C. Hwang, P. S. Lakey, A. Zuend, J. P. Abbatt and M. Shiraiwa, Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(24), 11658–11663, DOI:10.1073/pnas.1902517116.
- J. Wang, Z. Chen and B. Chen, Adsorption of Polycyclic Aromatic Hydrocarbons by Graphene and Graphene Oxide Nanosheets, Environ. Sci. Technol., 2014, 48(9), 4817–4825, DOI:10.1021/es405227u.
- S. N. Chu, S. Sands, M. R. Tomasik, P. S. Lee and V. F. McNeill, Ozone Oxidation of Surface-Adsorbed Polycyclic Aromatic Hydrocarbons: Role of PAH−Surface Interaction, J. Am. Chem. Soc., 2010, 132(45), 15968–15975, DOI:10.1021/ja1014772.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ea00024b |
| This journal is © The Royal Society of Chemistry 2024 |