Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green multicomponent synthesis of pyrano[2,3-c]pyrazole derivatives: current insights and future directions
Afrisham Ahmad ,
Sithara Rao
,
Sithara Rao and
Nitinkumar S. Shetty
and
Nitinkumar S. Shetty *
*
Department of Chemistry, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India. E-mail: nitin.shetty@manipal.edu
First published on 2nd October 2023
Abstract
The past decade has witnessed significant progress in synthesizing structurally diverse and biologically relevant pyrano[2,3-c]pyrazole derivatives through the integration of green methodologies. This review summarizes the recent advances in the green multicomponent synthesis of pyrano[2,3-c]pyrazole and spiro-pyrano[2,3-c]pyrazole derivatives. These include the application of energy-efficient techniques such as microwave and ultrasound-assisted synthesis, benign catalysts and biodegradable composites, solvent selection with a focus on water as a renewable and non-toxic medium, and solvent-free conditions. The review consolidates the current knowledge and future research directions, providing a valuable resource for researchers dedicated to advancing green chemistry practices.
1 Introduction
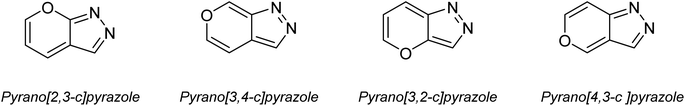
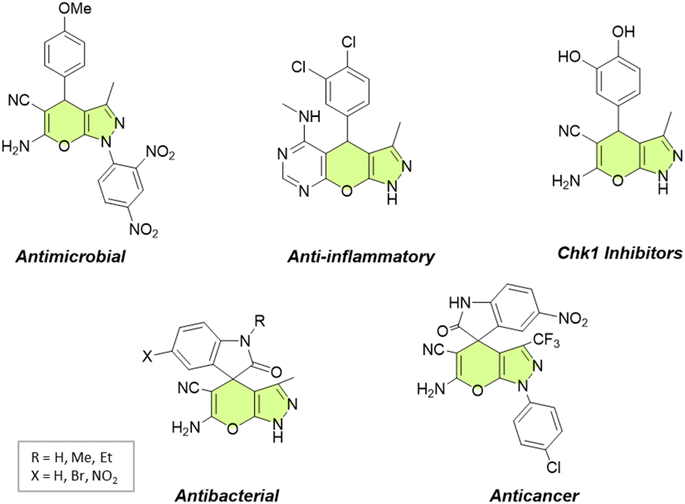
In recent years, the field of organic synthesis has witnessed a remarkable paradigm shift towards sustainability and environmental consciousness. This transformation is illustrated by the emergence of green chemistry, which advocates for the development of eco-friendly and resource-efficient synthetic methodologies.1,2 Among the myriad reactions and strategies in organic synthesis, the multicomponent synthesis of heterocyclic compounds holds a prominent position due to its efficiency and versatility.3Pyranopyrazoles, as a subclass of heterocycles, have garnered significant attention owing to their diverse structural significance and biological activities.4–6 These compounds are composed of fused pyran and pyrazole rings, existing in four distinct isomer arrangements: pyrano[2,3-c]pyrazole, pyrano[3,2-c]pyrazole, pyrano[3,4-c]pyrazole, and pyrano[4,3-c]pyrazole (Fig. 1). However, pyrano[2,3-c]pyrazoles are the most extensively investigated due to the biological significance of this isomer. These compounds have shown promising antimicrobial,7,8 anticancer,9 anti-inflammatory,10 and antiviral properties.11 Additionally, they exhibit the capability to potentially inhibit the activity of the human Chk1 kinase enzyme12 (Fig. 2). Their structural diversity allows for the modulation of activity by modifying different regions of the molecule, opening up possibilities for structure–activity relationship studies.13
The synthesis of pyrano[2,3-c]pyrazole has, undeniably, been a subject of considerable research efforts, yielding numerous methods and synthetic routes. Yet, the existing body of literature predominantly focuses on achieving high yields and product diversity, often overshadowing the critical importance of sustainability. Traditional methods for their synthesis often require multiple reactions and purification steps in harsh reaction conditions, such as high temperatures or strong acids, leading to low yields and potential side reactions. Furthermore, the use of toxic solvents, hazardous reagents, and high energy consumption contribute to environmental pollution, waste generation, and carbon emissions.2 While the exploration of various reaction pathways and synthetic strategies is undoubtedly essential, it is equally imperative to acknowledge the environmental impact of these processes. The past decade has witnessed significant progress in this area, with researchers developing innovative strategies and employing green principles to access pyranopyrazoles efficiently.14,15 Among the green techniques in organic chemistry are reactions involving solid-supported, bio- and asymmetric catalysis and synthesis,16,17 water and other green solvents,2 ionic liquids (ILs) or without solvents, microwave, ultrasound, ultraviolet (UV), and flow reactors.18,19
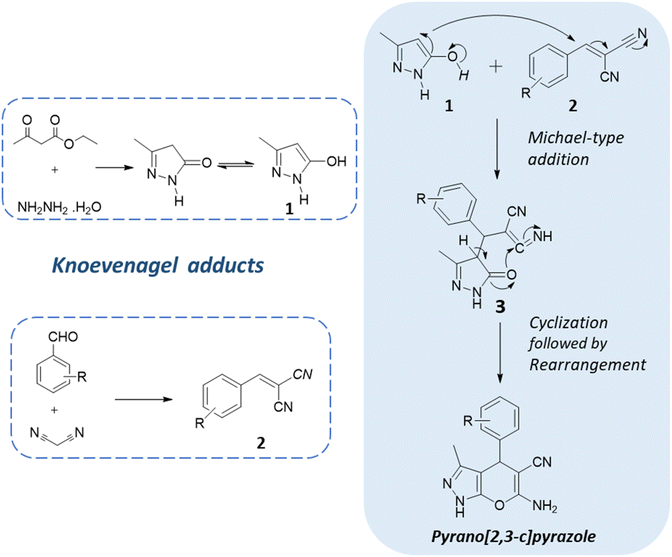
One of the key strategies employed in the synthesis of pyranopyrazoles is multicomponent reactions. These are one-pot reactions that involve the sequential addition of multiple reagents and catalysts, enabling the rapid assembly of the target molecules in a single reaction vessel.20,21 The general reaction scheme of the one-pot multicomponent reaction of pyranopyrazoles typically involves an aldehyde, malononitrile, a β-ketoester/ethyl acetoacetate, hydrazine hydrate, and an appropriate catalyst or promoter.22 The reaction proceeds through a series of sequential transformations, including condensation, cyclization, and subsequent rearrangement, yielding the pyranopyrazole product (Fig. 3). MCRs often proceed under mild reaction conditions, minimizing the need for harsh reagents.23,24 They offer several advantages, including atom economy, step economy, and the simultaneous assembly of multiple building blocks and thus comply with the principles of green chemistry. Furthermore, the one-pot nature of this reaction reduces the number of purification steps required, minimizing potential side reactions and simplifying the overall synthetic process.25,26
Numerous preceding review articles have appropriately lauded the progress in pyranopyrazole synthesis through MCRs,27,28 showcasing ingenious strategies, high yields, and novel applications. In this landscape, our review article aims to stand apart by offering a fresh perspective on the synthesis of pyranopyrazoles through MCRs, one that prioritizes the principles of green chemistry.
In this comprehensive review article, we have meticulously examined a decade's worth of research papers, spanning from 2012 to 2023, in order to provide a holistic overview of the advancements made in the green multicomponent synthesis of pyrano[2,3-c]pyrazoles and spiro-pyrano[2,3-c]pyrazole derivatives. Our primary objective was to focus on research that not only explored various synthetic routes but also adhered to the fundamental principles of green chemistry. Instead of limiting our scope to a single green chemistry principle, we sought out studies that harmoniously integrated multiple eco-friendly strategies, for instance, the application of energy-efficient techniques such as microwave and ultrasound-assisted synthesis, catalyst design using environmentally friendly metals and biodegradable composites, solvent selection with a focus on water as a renewable and non-toxic medium, and solvent-free conditions. This discerning approach allowed us to select and showcase papers that exemplified the multifaceted nature of sustainable synthesis. In our review, we have placed particular emphasis on elucidating the key findings and novel methodologies outlined in these selected papers. Through this extensive exploration, we aim to offer readers a comprehensive and insightful understanding of the green multicomponent synthesis of pyranopyrazoles, while highlighting the pivotal role of sustainable chemistry in shaping the future of organic synthesis.
2 Physical methods
Energy inputs play a crucial role in organic synthesis, influencing reaction rates, yields, selectivity, and overall process efficiency. Conventional heating supplies the necessary energy to surmount activation barriers. However, to maintain a balanced energy system during prolonged reaction times, a cooling medium such as a water reflux condenser is essential for the efficient transfer of thermal energy.29 Reaction temperatures can be high, which may cause undesired side reactions that can be less sustainable compared to green approaches like microwave heating, ultrasound irradiation, concentrated solar radiation, etc. These alternative energy inputs are characterized by their potential to reduce energy consumption, minimize waste, and promote sustainable practices. They can also lead to shorter reaction times, higher yields, and improved product selectivity.302.1 Microwave-assisted technique
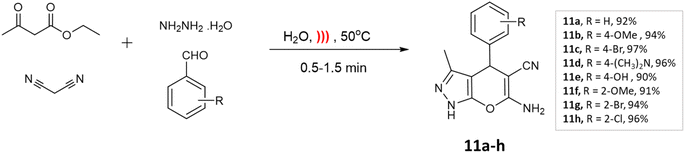
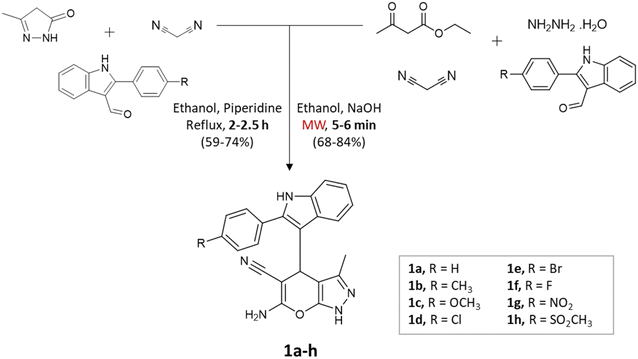
Microwave irradiation provides rapid and selective heating of reaction mixtures using electromagnetic waves. They accelerate reactions due to direct interaction with polar molecules and lead to shorter reaction times as well as increased yields making the process energy-efficient.31,32Kathrotiya et al.33 synthesized a series of indol-3-yl substituted pyrano[2,3-c]pyrazoles using two different methods: a conventional three-component reaction under reflux conditions and a four-component reaction, with the assistance of microwave irradiation (Scheme 1). In the three-component reaction, 2-phenyl-1H-indole-3-carbaldehydes, malononitrile, and 3-methyl-1H-pyrazol-5(4H)-one were condensed in ethanol with piperidine. The reaction mixture was gradually heated and refluxed for 2–2.5 h. On the other hand, the four-component reaction involved the condensation of 2-phenyl-1H-indole-3-carbaldehydes, ethyl acetoacetate, malononitrile, and hydrazine hydrate in ethanol with NaOH as the catalyst. Microwave irradiation at an output power of 280 W was applied to the mixture for a period of 5–6 min. A comparative analysis of the two methods revealed that microwave irradiation proved to be more effective in accelerating the reactions.
 | ||
| Scheme 1 Synthesis of indol-3-yl substituted pyrano[2,3-c]pyrazoles using conventional and microwave-assisted methods. | ||
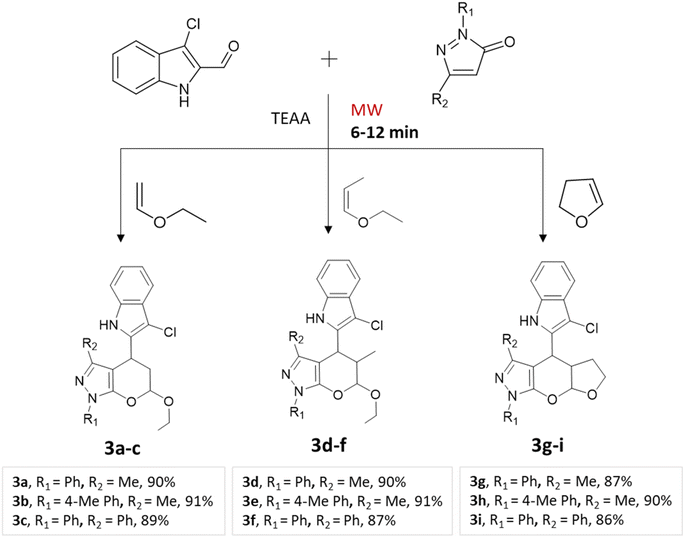
A regio- and stereoselective method for synthesizing heteroaryl pyranopyrazoles was developed by J. Parmar and colleagues.34 The procedure involved a multi-component domino reaction using indole- or quinolcarbaldehyde, pyrazolone, and enol ethers in triethylammonium acetate (TEAA) under the influence of microwave irradiation (Schemes 2 and 3). To optimize the reaction conditions, different refluxing solvents (acetylene, xylene, toluene, TEAA) were used. It was found that ionic liquid TEAA as a reaction medium required no catalyst and yielded 88% of the desired products in 5 h. By employing microwave irradiation, the reaction time was further reduced to just 10 min.
P. Shukla and colleagues35 prepared a range of pyrano[2,3-c]pyrazole using two different methods: conventional heating and microwave-assisted multicomponent approach, involving ethyl acetoacetate, hydrazine, malononitrile, and aldehydes using triethylamine base (Scheme 4). Assessing the two approaches according to yields obtained and reaction completion times, the researchers noted that although the conventional heating method yielded slightly superior results in terms of product yields, the microwave-assisted synthesis notably and substantially reduced reaction durations.
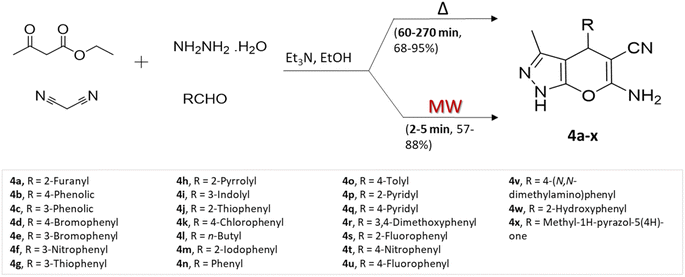 | ||
| Scheme 4 Synthesis of pyrano[2,3-c]pyrazoles using traditional heating and microwave-assisted techniques. | ||
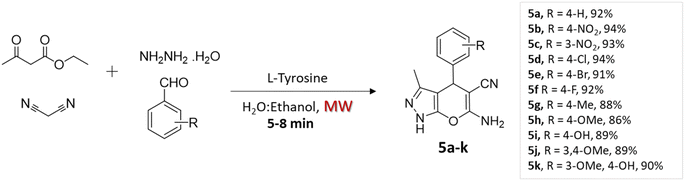
Rupnar et al.36 employed the combination of microwave irradiation and an eco-friendly solvent (H2O–ethanol) to facilitate the synthesis of pyrano[2,3-c]pyrazole derivatives. This innovative approach involved the four-component condensation of acetoacetic ester, hydrazine hydride, aldehydes, and malononitrile, in the presence of L-tyrosine (Scheme 5).
M. S. Vasava et al.37 successfully synthesized biologically active heterocyclic scaffolds based on pyrano[2,3-c]pyrazole using an MCR approach. Various substituted aldehyde derivatives were combined with 2,4-dinitrophenyl hydrazine, ethyl acetoacetate, and malononitrile using SnCl2 as the catalyst (Scheme 6). Two methods were compared: conventional heating and microwave irradiation. In the conventional method at 80 °C, the reaction took 1.4 h and resulted in an 80% yield. However, the microwave irradiation method produced the desired product in just 25 min, with an 88% yield.
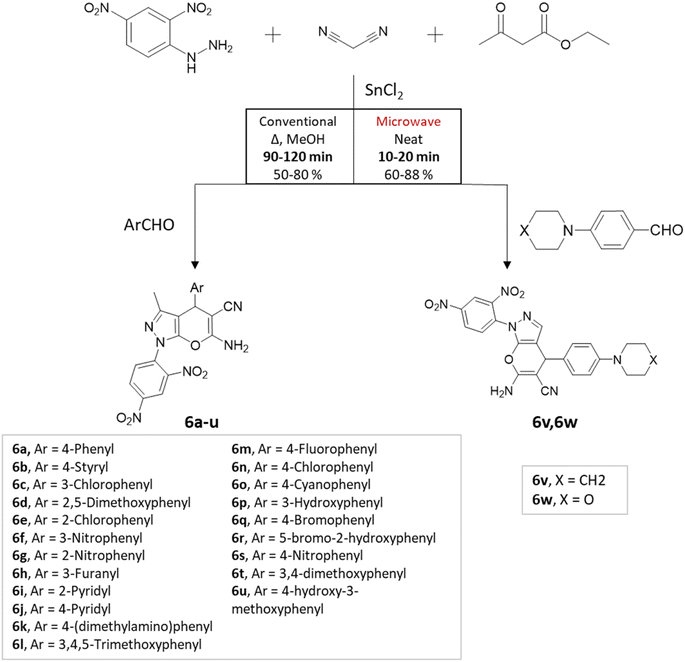 | ||
| Scheme 6 Pyrano[2,3-c] pyrazole synthesis using traditional heating and microwave-assisted techniques. | ||
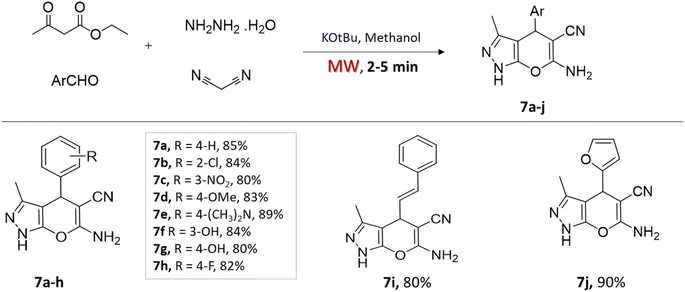
In a very recent study by Yallappa et al.,38 potassium t-butoxide, a base catalyst, was employed in a one-pot four-component approach to synthesize various 4H-pyrano[2,3-c]pyrazoles (Scheme 7). The condensation reaction involves a mixture of ethyl acetoacetate, hydrazine hydrate, malononitrile, and aromatic aldehydes in the methanol solvent with a catalytic amount of KOtBu. Microwave irradiation led to faster reaction completion (less than 5 min) and excellent yields for the synthesized compounds compared to conventional stirring at room temperature (Table 1).
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Indol-3-yl substituted pyrano[2,3-c]pyrazoles | (a) Three-component system: 2-phenyl-1H-indole-3-carbaldehydes, malononitrile, and 3-methyl-1H-pyrazole-5(4H)-one | Piperidine | Ethanol | Conventional reflux | 2–2.5 h | 59–74% | Kathrotiya et al.33 |
| (b) Four-component system: 2-phenyl-1H-indole-3-carbaldehydes, ethyl acetoacetate, malononitrile, and hydrazine hydrate | NaOH | Microwave | 5–6 min | 68–84% | |||
| Quinolylpyrano[2,3-c]pyrazoles and indolylpyrano[2,3-c]pyrazoles | Three-component system: indole- or quinol carbaldehyde, pyrazolone, and enol ethers | — | Triethyl-ammonium acetate (TEAA) | Microwave | 6–12 min | 82–92% | J. Parmar et al.34 |
| Substituted pyrano[2,3-c]pyrazoles | Four-component system: acetoacetic ester, hydrazine hydride, aldehydes, and malononitrile | L-Tyrosine | H2O–ethanol | Microwave | 5–8 min | 86–94% | D. Rupnar et al.36 |
| Pyrano[2,3-c]pyrazoles | Four-component system: ethyl acetoacetate, hydrazine, malononitrile, and aldehydes | Triethylamine | Ethanol | Conventional reflux | 1–4.5 h | 68–95% | P. Shukla et al.35 |
| Microwave | 2–5 min | 57–88% | |||||
| Pyrano[2,3-c]pyrazoles | Four-component system: substituted aldehyde, 2,4-dinitrophenyl hydrazine, ethyl acetoacetate, and malononitrile | SnCl2 | Methanol | Conventional reflux | 1.5–2 h | 50–80% | S. Vasava et al.37 |
| Solvent-free | Microwave | 10–20 min | 60–88% | ||||
| 4H-Pyrano[2,3-c] pyrazoles | Four-component system: ethyl acetoacetate, hydrazine hydrate, malononitrile, and aromatic aldehydes | Potassium t-butoxide (base) | Methanol | Microwave | 2–5 min | 80–90% | Yallappa et al.38 |
While microwave-assisted heating significantly reduces reaction times, most reactions still yielded comparable results to those achieved with conventional reflux heating. It is worth noting that there were instances where the yield was not as substantial. Hence, as a note to future research, it is essential to consider all factors, as they may not depend solely on the heating technique, but also on variables such as substituents, solvents, and catalysts.
2.2 Concentrated solar radiation technique
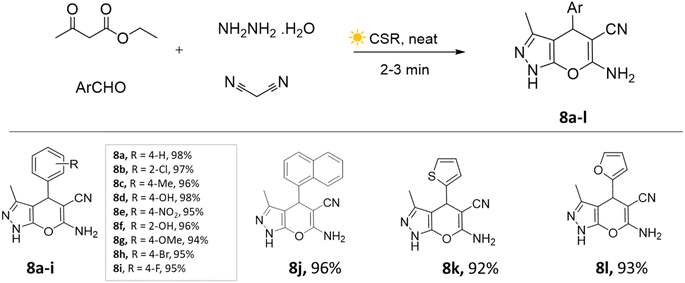
The concentrated solar radiation method involves focusing sunlight using a solar collector concentrated onto a reaction vessel with optical instruments and a temperature sensor to achieve the desired temperature for a chemical reaction. However, there is limited control over reaction temperature owing to the dependence on sunlight availability. These are also specific to certain reactions and geographical locations.39,40Yatin U. Gadkari et al.,41 showcased the utilization of concentrated solar radiation for the synthesis of pyranopyrazole derivatives. This involved a solvent-free and catalyst-free approach employing a multi-component strategy. The aldehyde, ethyl acetoacetate, malononitrile, and hydrazine hydrate mixture was placed in a round-bottom flask and continuously stirred on a magnetic stirrer under concentrated solar radiation. The precipitate was observed within 3–4 min (Scheme 8). This method resulted in remarkable energy savings of approximately 98% compared to the conventional approach, while also exhibiting exceptional speed and high yields.
Despite having numerous advantages and significant environmental importance, the CSR method has not received extensive research attention. Therefore, efforts should be directed toward comprehending its characteristics to harness its complete potential.
2.3 Ultrasound irradiation technique
Ultrasonic waves induce cavitation, leading to the formation and collapse of bubbles, which create localized high temperatures and pressures, enhancing reaction rates. It reduces the need for elevated temperatures and potentially hazardous reagents while improving selectivity and purity due to milder reaction conditions.42,43An efficient four-component synthesis of dihydropyrano[2,3-c]pyrazole derivatives using ultrasound irradiation was reported by Ablajan et al.44 The desired compounds were successfully synthesized with favorable to exceptional yields using a ceric ammonium nitrate (CAN) catalyst in a water medium under the influence of ultrasound irradiation (Scheme 9).
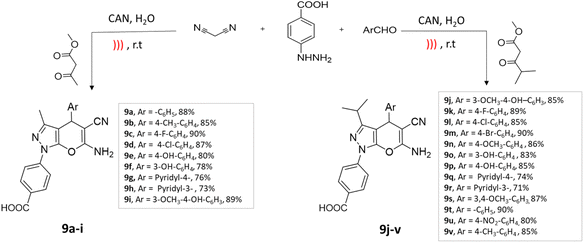 | ||
| Scheme 9 Synthesis of dihydropyrano[2,3-c]pyrazole derivatives using CAN under ultrasound-mediated technique. | ||
Dandia and coworkers45 utilized ZnS nanoparticles within a water environment under ultrasonic irradiation. The researchers conducted a one-pot three-component synthesis involving isatin, ethyl-cyanoacetate, and 3-methyl-1-phenyl-2-pyrazolin-5-one to successfully synthesize spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives (Scheme 10). The catalyst could be reused for up to three cycles.
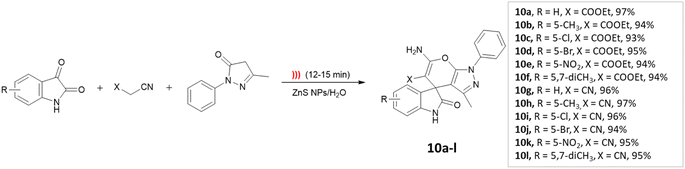 | ||
| Scheme 10 Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives using ZnS NPs under ultrasonic radiation. | ||
Shabalala et al.46 reported the pyrano[2,3-c]pyrazole synthesis through a catalyst-free multicomponent reaction. This reaction involved ethyl acetoacetate, aromatic aldehydes, hydrazine monohydrate, and malononitrile in a water medium, facilitated by ultrasonic irradiation, yielding excellent results (Scheme 11).
Maddila and colleagues47 employed Mn/ZrO2 in the ultrasound-assisted synthesis of pyrano[2,3-c]pyrazole-3-carboxylate and pyrano[2,3-c]pyrazole-5-carbonitriles. This process entailed coupling reactions of ethyl acetoacetate or dimethylacetylenedicarboxylate, hydrazine hydrate, aromatic aldehyde, and malononitrile in an aqueous ethanol solution (Scheme 12). Under ultrasonication, a yield of 98% is obtained within 10 min, compared to an 83% yield achieved by conventional methods in 1 h (Table 2).
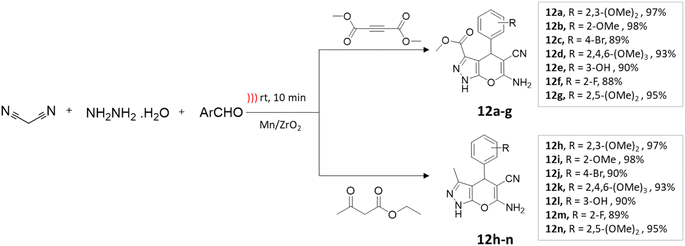 | ||
| Scheme 12 Synthesis of pyrano[2,3-c]pyrazole derivatives using Mn/ZrO2 under ultrasound-mediated technique. | ||
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Dihydropyrano[2,3-c]pyrazoles | Four-component system: 4-hydrazinobenzoic acid, β-keto esters, aromatic aldehydes, and malononitrile | Ceric ammonium nitrate (CAN) | Water | Ultrasound | 45–60 min | 70–90% | Ablajan et al.44 |
| Spiro[indoline-3,4′-pyrano[2,3-c]]pyrazoles | Three-component system: isatin, ethyl-cyanoacetate, and 3-methyl-1-phenyl-2-pyrazolin-5-one | ZnS nanoparticles | Water | Ultrasound | 12–15 min | 93–97% | Dandia et al.45 |
| Pyrano[2,3-c]pyrazoles | Four-component system: aromatic aldehydes, hydrazine monohydrate, ethyl acetoacetate, and malononitrile | Catalyst free | Water | Ultrasound | 30–90 s | 90–97% | Shabalala et al.46 |
| Pyrano[2,3-c]pyrazole-3-carboxylate and pyrano[2,3-c]pyrazole-5-carbonitriles | Four-component system: dimethylacetylenedicarboxylate/ethyl acetoacetate, hydrazine hydrate, malononitrile, and aromatic aldehyde | Mn/ZrO2 | Aq. ethanol | Ultrasound | 10 min | 88–99% | Maddila et al.47 |
For the Ultrasound-assisted reactions, we witness shortened reaction durations, with aqueous media commonly employed as the solvent, rendering these reactions environmentally friendlier in multiple aspects. However, there remains ample room to investigate the impact of Ultrasonic irradiation further in the organic synthesis of pyrano-pyrazoles, particularly concerning their medicinal and biological properties.
3 Catalysis in green processes
In organic synthesis, catalysis offers several advantages, including increased reaction rates, enhanced selectivity, and milder reaction conditions. It plays a pivotal role in reducing energy consumption, minimizing waste, and improving overall process efficiency. The key difference between conventional and green catalysis lies in their environmental impact. Conventional catalysts may involve toxic or costly materials, while green catalysts emphasize sustainability, utilizing biodegradable, renewable, or benign substances, resulting in more eco-friendly and efficient organic synthesis processes.30,48,493.1 Nano-catalysis
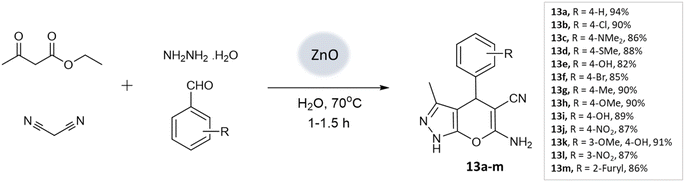
Nano-catalysis has evolved to provide rapid and sustainable routes, reducing waste and increasing reusability due to their high surface-to-volume ratio, allowing greener and more efficient synthesis of diverse heterocyclic structures.50 They often exhibit high selectivity, allowing for precise control over the desired reaction pathways, thereby minimizing the formation of unwanted byproducts. Furthermore, they are highly stable and durable, withstanding harsh reaction conditions and prolonged use without significant loss of activity.16,51,52S. U. Tekale and coworkers53 documented a method for synthesizing 4H-pyrano[2,3-c]pyrazoles, utilizing a zinc oxide nanoparticle-catalyzed multicomponent water-based reaction. The crystalline structure of the ZnO nanoparticles was confirmed through XRD investigations. Moreover, TEM analysis unveiled particle sizes spanning from 50 to 100 nm, creating an extensive surface area that facilitated the accelerated formation of the desired products. Employing an aqueous medium, a four-component coupling reaction involving ethyl acetoacetate, aromatic aldehyde, malononitrile, and hydrazine hydrate, along with ZnO nanoparticles as a catalyst, resulted in the production of pyranopyrazoles with elevated yields in a brief timeframe (Scheme 13).
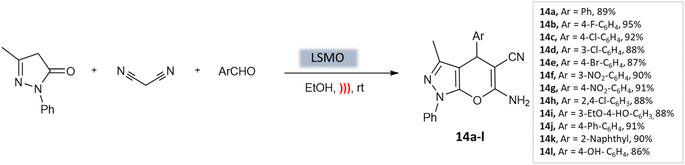
Azarifar et al.54 developed highly efficient magnetic lanthanum strontium magnesium oxide (La0.7 Sr0.3MnO3 or LSMO) nanoparticles with remarkable swiftness. Magnetic nanoparticles (MNPs) are easily accessible, enabling their widespread use due to their stable catalyst linkages. Additionally, their simple separation using an external magnetic field streamlines the purification process. Moreover, they exhibit lower catalyst leaching compared to other material-supported catalysts, making them a highly promising choice for catalytic applications.55,56 The composite catalyst here, La0.7 Sr0.3MnO3, demonstrated outstanding characteristics such as a surface area of 39 m2 g−1, an average size of approximately 20 nm, and a magnetization of around 15 emu g−1. Using just 5 mol% of LSMO catalyst under ultrasound irradiation in an ethanol medium, researchers achieved high efficiency in producing pyrano-[2,3-c]-pyrazole scaffolds (Scheme 14). This process yielded excellent yields within 10 min at room temperature.
El-Remaily et al.57 explored the use of magnetic Fe3O4 nanoparticles as a heterogeneous catalyst in the synthesis of pyranopyrazoles. This involved a four-component reaction, wherein a combination of ethyl acetoacetate, hydrazine hydrate, aldehydes or ketones, and malononitrile was reacted in a water medium at room temperature (Scheme 15). The best yields were obtained with 6 mol% Fe3O4-MNPs in aqueous media within 15 min. The catalyst could be reused up to fourteen times with no significant loss of catalytic activity.
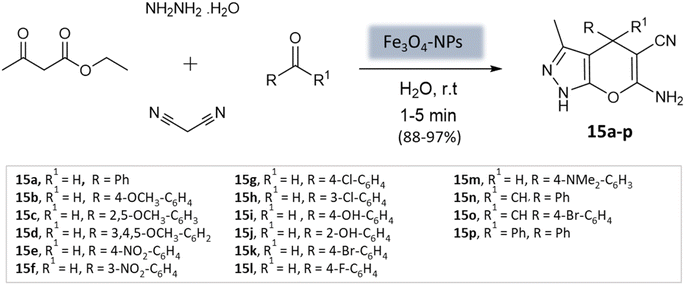 | ||
| Scheme 15 Magnetic Fe3O4 nanoparticles catalyzed four-component synthesis of pyranopyrazoles in aqueous medium. | ||
Pradhan et al.58 successfully synthesized a highly efficient nanocatalyst called copper ferrite (CuFe2O4) using a straightforward citric acid complex method. This catalyst demonstrated effectiveness in synthesizing pyrano[2,3-c]-pyrazoles through a four-component reaction involving alkyl nitrile derivatives, various hydrazine derivatives, dialkyl acetylenedicarboxylate, and ethyl acetoacetate (Scheme 16). Notably, using just 8 mol% of CuFe2O4, the researchers achieved remarkable yields in water at 60 °C within 4 h. However, when the ethyl acetoacetate was replaced with dialkyl acetylenedicarboxylate, the desired product yields were unsatisfactory (12–43%).
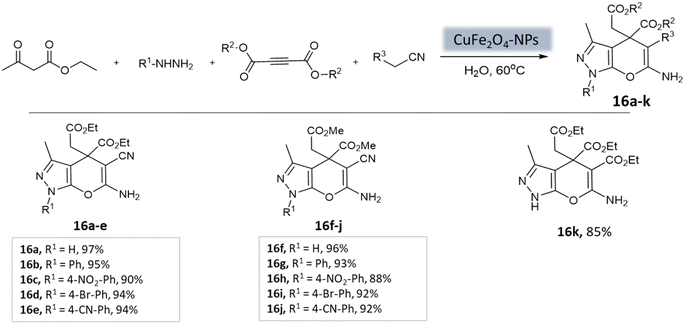 | ||
| Scheme 16 CuFe2O4 nanoparticles catalyzed synthesis of 3-methyl-1,4-dihydropyrano[2,3-c]pyrazole derivatives in aqueous medium. | ||
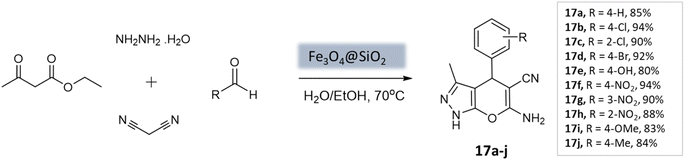
Soleimani et al.59 developed Fe3O4@SiO2 core–shell nanoparticles as a magnetically separable nanocatalyst for a four-component coupling reaction. This reaction involved the condensation of aromatic aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate in H2O/EtOH mixture to produce substituted pyranopyrazoles in high yields within 40 min (Scheme 17). The Fe3O4@SiO2 NPs had a roughly spherical morphology with some agglomeration. XRD analysis confirmed that the silica-coated iron oxide NPs retained the magnetic properties of the bare Fe3O4 NPs. The catalyst demonstrated durability and could be reused up to five times without significant loss in catalytic activity.
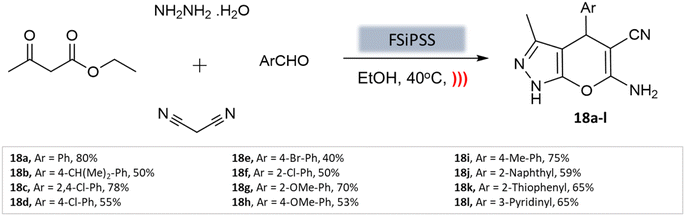
A highly efficient and recoverable nanomagnetic catalyst, Fe3O4@SiO2@OSi(CH2)3–N(3-pyridoyl sulfonic acid) semicarbazide (FSiPSS), was designed, synthesized, and characterized using various techniques by Beiranvand et al.60 for the synthesis of diverse pyranopyrazole derivatives through a one-pot four-component condensation reaction of ethyl acetoacetate, hydrazine hydrate, aromatic aldehydes, malononitrile under ultrasonication (Scheme 18). The catalyst's specific surface area was found to be 35.6 m2 g−1 with an average size between 13.66 and 35.86 nm to facilitate the catalyst's effectiveness in carrying out the desired synthesis. The reaction achieved very short reaction times, good to high yields, and easy work-up. This novel nanomagnetic catalyst shows great potential for efficient and sustainable synthesis processes.
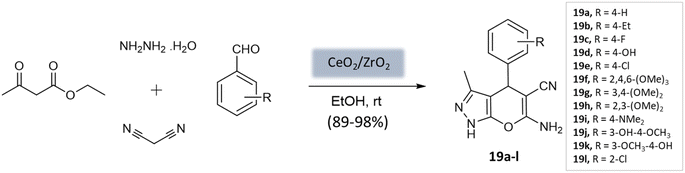
Maddila et al.61 introduced a ceria-doped zirconia catalyst prepared via the wet impregnation method for the synthesis of pyranopyrazoles with remarkable yields within 15 min at room temperature (Scheme 19). The four-component reaction, involving hydrazine hydrate, ethyl acetoacetate, malononitrile, and substituted aldehydes in ethanol, was efficiently catalyzed using CeO2/ZrO2. The catalyst could be easily recovered through filtration and recycled for up to six cycles while maintaining its efficiency.
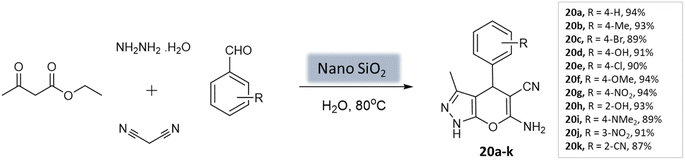
Patel et al.62 utilized a recyclable nano-SiO2 catalyst to prepare pyrano[2,3-c]-pyrazoles. The catalyst was synthesized from wheat straw agricultural waste through the sol–gel process. The catalyst exhibited a uniform distribution and a spherical shape, with a crystallite size ranging from 100 to 200 nm. BET analysis revealed important properties, including a surface area of 215.6 m2 g−1, a pore volume of 0.269 cm3 g−1, and a pore diameter of 7.1 nm. The reaction involved hydrazine hydrate, malononitrile, aromatic aldehydes, and ethyl acetoacetate in water as a multi-component system. Notably, using only 10 mol% of the nanocatalyst yielded the best performance, achieving excellent yields within 40 s (Scheme 20). The catalyst remained stable for up to five runs without a significant decrease in activity.
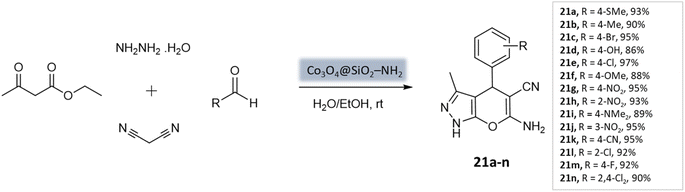
Shakiba Shahbazi et al.63 developed an efficient method to prepare dihydropyrano[2,3-c]pyrazoles via a multicomponent reaction of aryl aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate in the presence of SiO2@(3-aminopropyl)triethoxysilane-coated cobalt oxide (Co3O4) nanocomposite as the catalyst (Scheme 21). The nanocomposite had a cloudy and spherical shape, as observed in the FE-SEM image. Excellent yields and quick reaction times were obtained from the reaction, which can be attributed to the Bronsted–Lowry base's strong catalytic activity and high surface-to-volume ratio.
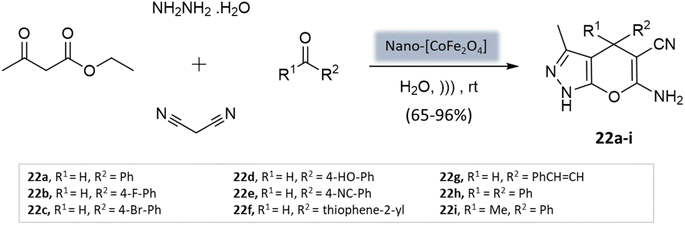
Mishra et al.64 presented a novel method for synthesizing pyranopyrazole scaffolds employing nanomagnetic iron material as a reusable catalyst in an aqueous solvent under the ultrasonication technique. The protocol involved the condensation of malononitrile, hydrazine hydrate, ethyl acetoacetate, and substituted aldehydes with the CoFe2O4 catalyst (Scheme 22). Remarkably, both electron-withdrawing and electron-donating groups exhibited good reactivity and provided significant yields of the desired products.
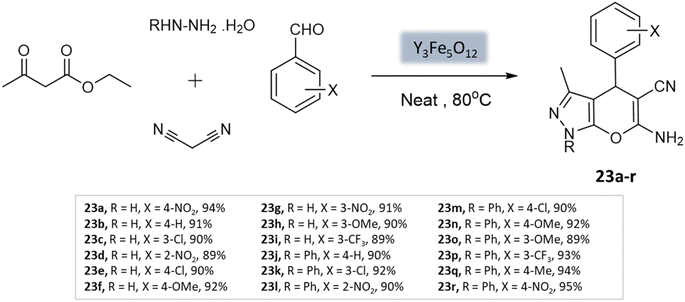
Sedighinia et al.65 introduced a highly efficient and recyclable nanocatalyst called yttrium iron garnet (Y3Fe5O12; YIG). This catalyst was utilized for the synthesis of pyranopyrazoles through the combination of hydrazine hydrate, ethyl acetoacetate, malononitrile, and substituted aldehydes under solvent-free conditions at 80 °C. The reaction exhibited excellent yields within a short duration of 20 min (Scheme 23). The nanocatalyst could be easily recycled and maintained its activity for up to eight runs.
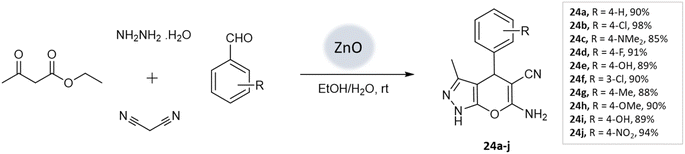
Prakash Chhattise et al.66 used a hydrothermal technique to synthesize nanostructured ZnO. The catalytic activity of this nanostructured ZnO was evaluated as a heterogeneous catalyst in the multicomponent synthesis of pyranopyrazole derivatives (Scheme 24). XRD analysis confirmed the formation of highly crystalline ZnO with a wurtzite structure. FESEM analysis revealed the formation of submicron-sized spherical structures resembling marigold flowers. Remarkable yields were achieved within 15–30 min (Table 3).
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| 4H-Pyrano[2,3-c]pyrazoles | Four-component system: aromatic aldehyde, malononitrile, ethyl acetoacetate, and hydrazine hydrate | Zinc oxide NPs | Water | Reflux, heating (70 °C) | 1–1.5 h | 82–94% | U. Tekale et al.53 |
| Pyrano-[2,3-c]-pyrazoles | Three-component system: malononitrile, different aromatic aldehydes, and 3-methyl-pyrazolone | Lanthanum strontium magnesium oxide (MNPs) | Ethanol | Ultrasound | 10 min | 87–95% | Azarifar et al.54 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: hydrazine hydrate, ethyl acetoacetate, aldehydes/ketones, and malononitrile | Magnetic Fe3O4 MNPs | Water | Room temperature stirring | 1–5 min | 88–97% | El-Remaily et al.57 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: alkyl nitrile derivatives, various hydrazine derivatives, dialkyl acetylene dicarboxylate, and ethyl acetoacetate | Copper ferrite (CuFe2O4) | Water | Stirring (60 °C) | 4 h | 88–97% | Pradhan et al.58 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: aromatic aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate | Fe3O4@SiO2 core–shell MNPs | Water/EtOH | Reflux, heating (70 °C) | 40 min | 80–94% | Soleimani et al.59 |
| Pyrano-[2,3-c]-pyrazole derivatives | Four-component system: benzaldehydes, pyrazolones, and malononitriles | Fe3O4@SiO2@OSi(CH2)3–N(3-pyridoyl sulfonic acid) semicarbazide MNPs | EtOH | Ultrasound | 3–7 min | 40–80% | Beiranvand et al.60 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: malononitrile, hydrazine hydrate, ethyl acetoacetate, and substituted aldehydes | Ceria-doped zirconia (CeO2/ZrO2) NPs | EtOH | Room temperature reflux | 15 min | 89–98% | Maddila et al.61 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: hydrazine hydrate, malononitrile, aromatic aldehydes, and ethyl acetoacetate | SiO2 NPs | Water | Reflux, heating (80 °C) | 40 min | 87–94% | Patel et al.62 |
| Dihydropyrano[2,3-c]pyrazoles | Four-component system: aryl aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate | SiO2@(3-aminopropyl)triethoxysilane-coated cobalt oxide (Co3O4) nanocomposite | Water/EtOH | Room temperature reflux | 35–55 min | 86–95% | Shahbazi et al.63 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: malononitrile, hydrazine hydrate, ethyl acetoacetate, and substituted aldehydes | Nanomagnetic iron material [CoFe2O4] | Water | Ultrasound | 5 min | 65–96% | Mishra et al.64 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: hydrazine hydrate, malononitrile, ethyl acetoacetate, and substituted aldehydes | Yttrium iron garnet (Y3Fe5O12; YIG) | Solvent free | Reflux, heating (80 °C) | 20 min | 89–95% | Sedighinia et al.65 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: hydrazine hydrate, malononitrile, ethyl acetoacetate, and substituted aldehydes | Zinc oxide (ZnO) NPs | Water/EtOH | Room temperature reflux | 15–30 min | 85–98% | Chhattise et al.66 |
The eco-friendliness of nano-catalyst production relies on factors like selecting non-toxic materials and preparation techniques, impacting energy consumption.67 Recent research emphasizes creating safe, sustainable nano-catalysts through energy-efficient methods like microwave and ultrasound-assisted synthesis, solvent-free synthesis, template-directed synthesis, and more.68 Nevertheless, our primary focus is optimizing reaction parameters to increase pyranopyrazole yields using nano-catalysts tailored to specific applications, all within shorter reaction durations. Future investigations should prioritize cost-effective and environmentally benign metal/nanoparticle catalysts for pyranopyrazole synthesis, building upon the aforementioned references.
3.2 Organocatalysis
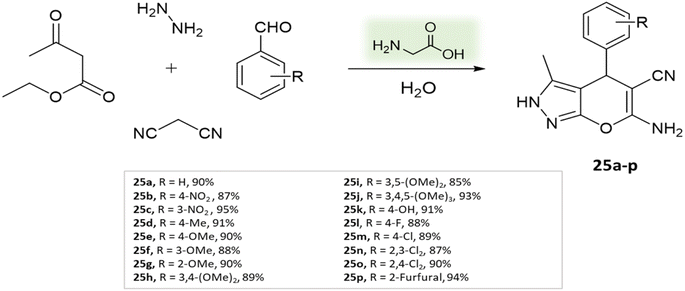
Organo-catalysis involves the use of small organic molecules as catalysts to facilitate chemical transformations. It's valuable in organic synthesis due to its compatibility with mild reaction conditions, often avoiding the need for transition metals. This approach offers advantages like atom economy, reduced environmental impact, and improved selectivity.69,70Madhusudana Reddy and colleagues71 used an easily accessible, non-toxic, and environmentally friendly catalyst Glycine to synthesize pyranopyrazoles from ethyl acetoacetate, hydrazine hydrate, aldehyde, and malononitrile in aqueous medium at 25 °C in 5–20 min (Scheme 25).
Zolfigol et al. employed biological organocatalyst isonicotinic acid under solvent-free conditions72 to synthesize 1,4-dihydropyrano[2,3-c]pyrazoles through a four-component condensation reaction involving ethyl acetoacetate, malononitrile, aryl aldehydes, and hydrazine hydrate, carried out at a temperature of 85 °C (Scheme 26). Remarkably, the catalyst retained its catalytic efficacy within the boundaries of experimental error for four consecutive runs.
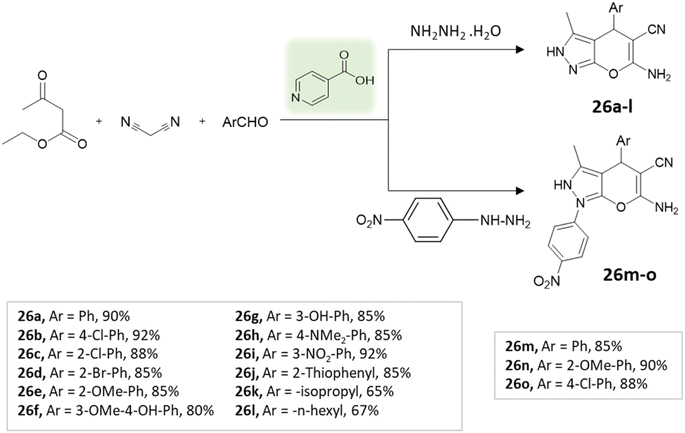
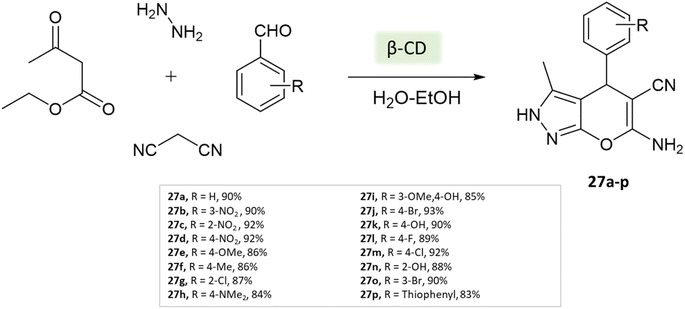
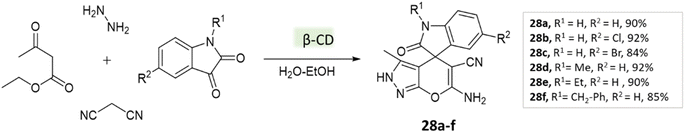
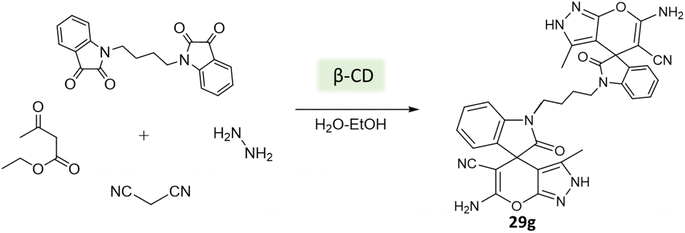
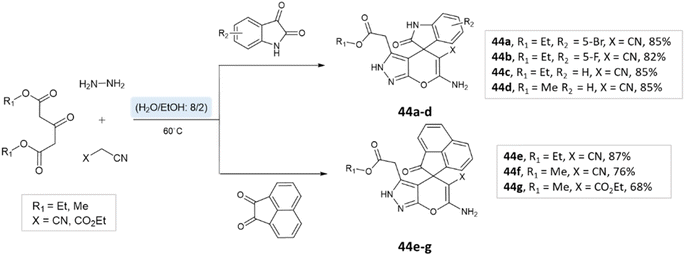
Tayade et al.73 utilized a biodegradable supramolecular β-cyclodextrin (β-CD) catalyst for the synthesis of pyrano[2,3-c]pyrazole and spiro-pyrano[2,3-c]pyrazole derivatives via MCR strategy. This process involved a reaction incorporating aldehydes, isatins, hydrazine hydrate, malononitrile, and β-ketoester, conducted in a mixture of H2O/EtOH at 80 °C (Schemes 27–29). Impressively, the catalyst retained its efficacy and could be reused for up to three cycles.
J. P. Sonar et al.74 employed sodium lactate as a catalyst within an aqueous ethanolic environment under reflux conditions to synthesize pyranopyrazoles. This process involved the combination of hydrazine hydrate, ethyl acetoacetate, malononitrile, and various aldehydes (Scheme 30). The approach is characterized by its simplicity and environmentally friendly nature, leading to the production of pyranopyrazoles with moderate to excellent yields in a short span of reaction time (Table 4).
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Pyrano-[2,3-c]-pyrazoles | Four-component system: ethyl acetoacetate, hydrazine hydrate, an aldehyde, and malononitrile | Glycine | Water | Room temperature stirring | 5–20 min | 85–94% | M. Reddy et al.71 |
| 1,4-Dihydropyrano[2,3-c]pyrazoles | Four-component system: malononitrile, hydrazine hydrate, ethyl acetoacetate, and aryl aldehydes | Isonicotinic acid | Solvent free | Reflux, heating (85 °C) | 10–15 min | 65–92% | Zolfigol et al.72 |
| Pyrano[2,3-c]pyrazole and spiro-pyrano[2,3-c]pyrazole derivatives | Four-component system: aldehydes, isatins, hydrazine hydrate, malononitrile, and β-ketoester | β-Cyclodextrin (β-CD) | Water/EtOH | Reflux, heating (80 °C) | 15–50 min | 83–93% | Tayade et al.73 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: hydrazine hydrate, malononitrile, ethyl acetoacetate, and substituted aldehydes | Sodium lactate | Water/EtOH | Room temperature reflux | 10–20 min | 67–91% | J. P. Sonar et al.74 |
Organo-catalysis stands out as the safest and most efficient method to synthesize pyranopyrazoles offering mild reaction conditions with impressive yields. It can be done in aqueous media, thus, eliminating the need for toxic solvents and reducing conventional reaction time. This eco-friendly approach warrants further exploration in advancing pyranopyrazole synthesis.
3.3 Bio-catalysis/natural catalysis
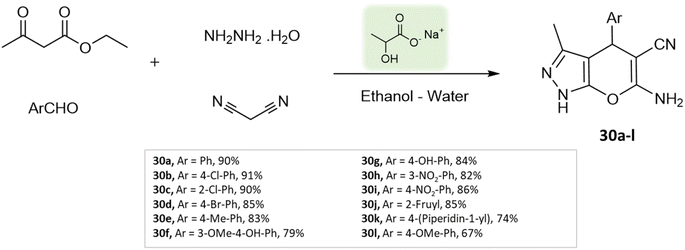
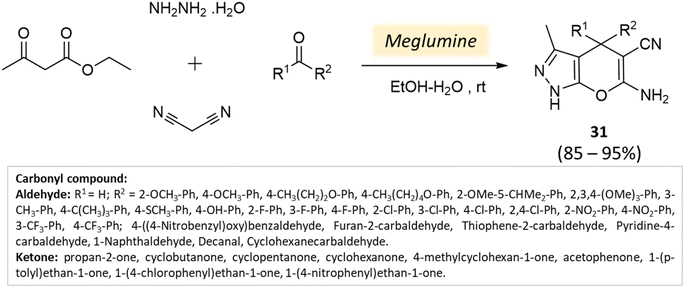
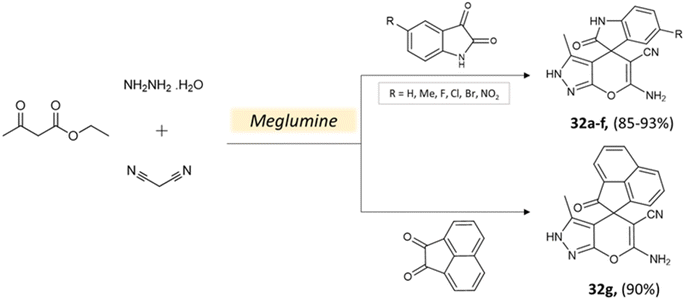
Bio-catalysis involves the use of natural catalysts like enzymes to drive chemical reactions and has gained prominence due to its specificity and compatibility with mild conditions, minimizing byproducts and waste. In organic synthesis, it offers regio- and stereoselectivity, enabling complex transformations.75Guo et al.76 used a bio-based chemical catalyst meglumine to develop a series of pyranopyrazoles and spiro[indoline-pyrano[2,3-c]-pyrazole] derivatives. A four-component reaction scheme was employed involving malononitrile, hydrazine hydrate, β-keto ester, and carbonyl compound or isatin in a solvent mixture of EtOH–H2O at room temperature (Schemes 31 and 32). The reaction, facilitated by 10 mol% meglumine, achieved excellent yields. The catalyst demonstrated reusability for up to 3 cycles with minimal loss of activity.
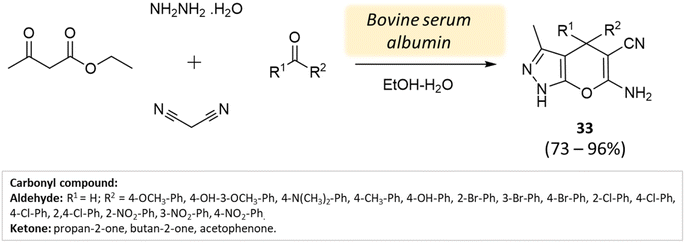
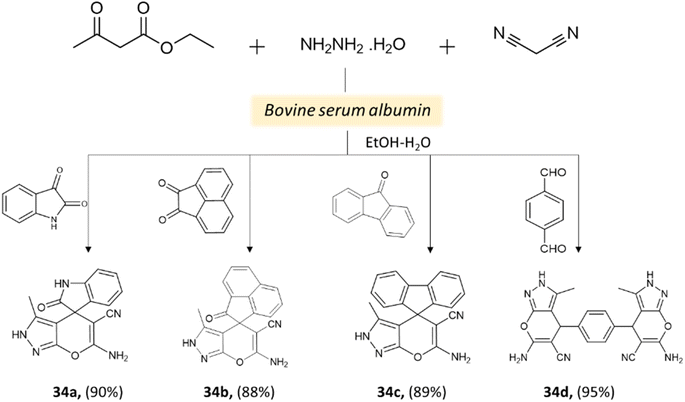
Xingtian and colleagues77 synthesized bovine serum albumin (BSA) and utilized it as a catalyst for generating pyrano[2,3-c]-pyrazole derivatives and spiro[indoline-pyrano[2,3-c]-pyrazole] derivatives. The catalytic performance of BSA was evaluated in a four-component reaction involving hydrazine hydrate, malononitrile, ethyl acetoacetate, and various carbonyl compounds. The reaction took place in an ethanol system at 45 °C for 45 min, resulting in excellent yields (Schemes 33 and 34). The recovered BSA could be reused for up to five cycles with alike effectiveness.
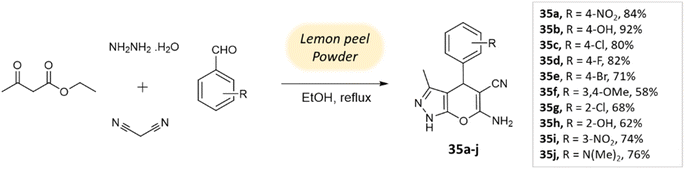
Ghodke et al.78 developed a facile method for synthesizing pyrano[2,3-c]pyrazoles via a component condensation reaction with lemon peel powder serving as a natural catalyst. In this process, malononitrile, aldehydes, ethyl acetoacetate, and hydrazine hydrate are reacted in ethanol under reflux conditions, with lemon peel powder (Scheme 35).
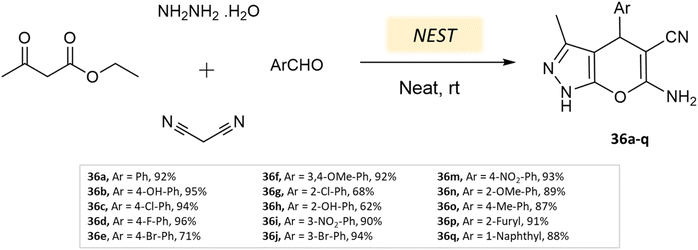
Arefeh Dehghani et al.79 effectively synthesized dihydropyrano[2,3-c]pyrazoles using nano-eggshell/Ti(IV) as a catalyst through a four-component reaction comprising ethyl acetoacetate, hydrazine hydrate, malononitrile, and aldehydes at room temperature under solvent-free conditions (Scheme 36). The technique offers notable advantages such as mild reaction conditions, quick reaction times, simple purification processes, excellent yields, the potential for catalyst reusability, and the removal of harmful organic solvents.
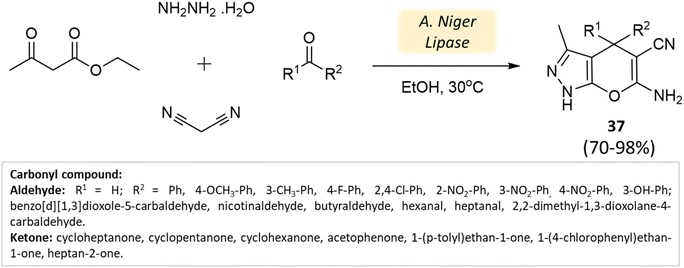
Bora and coworkers80 showcased the dihydropyran-[2,3-c]-pyrazole synthesis utilizing Aspergillus niger lipase as a catalyst. This lipase enzyme adeptly facilitated a four-component condensation reaction involving ethyl acetoacetate, hydrazine hydrate, malononitrile, and either aldehyde or ketone. Remarkable yields were achieved at a temperature of 30 °C (Scheme 37). Furthermore, the biocatalyst demonstrated reusability for up to three cycles.
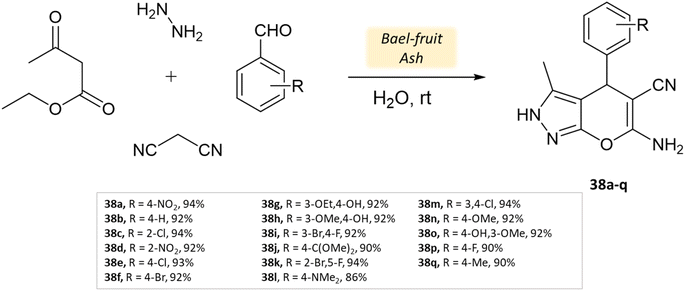
Shinde and his research team81 successfully developed a synthesis method using a natural catalyst made of bael fruit ash. Its efficacy was evaluated in synthesizing pyrano[2,3-c]-pyrazoles and pyrazolyl-4H-chromene derivatives in an aqueous medium through four-component reactions involving ethyl acetoacetate, malononitrile, hydrazine hydrate, various aldehydes. These reactions were conducted at room temperature for a duration of 30 min (Scheme 38). The method was notably suitable for producing pyrazolyl-4H-chromenes, yielding excellent results ranging from 86% to 94% for different salicylaldehydes (Table 5). Impressively, the BFA catalyst demonstrated stability across five cycles, exhibiting minimal loss of activity.
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction Time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Spiro[indoline-pyrano[2,3-c]-pyrazole] derivatives | Four-component system: malononitrile, hydrazine hydrate, β-keto ester, and carbonyl compound or isatin | Meglumine | Water/EtOH | Room temperature stirring | 25–40 min | 85–95% | Guo et al.76 |
| Pyrano[2,3-c]-pyrazole and spiro[indoline-pyrano[2,3-c]-pyrazole] derivatives | Four-component system: hydrazine hydrate, malononitrile, ethyl acetoacetate, and various carbonyl compounds | Bovine serum albumin (BSA) | Water/EtOH | Reflux, heating (45 °C) | 45 min | 73–96% | Xingtian et al.77 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate | Lemon peel powder | Ethanol | Room temperature reflux | 2–5 h | 58–92% | Ghodke et al.78 |
| Dihydropyrano[2,3-c]pyrazoles | Four-component system: aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate | Nano-eggshell/Ti(IV) | Solvent free | Room temperature stirring | 8–25 min | 62–96% | Dehghani et al.79 |
| Dihydropyrano[2,3-c]pyrazoles | Four-component system: aldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate | Aspergillus niger lipase (ANL) | Ethanol | Vigorous stirring, heating (30 °C) | 60 min | 70–98% | Bora et al.80 |
| Pyrano[2,3-c]-pyrazoles and pyrazolyl4H-chromene derivatives | Four-component system: aldehydes/salicaldehydes, malononitrile, ethyl acetoacetate, and hydrazine hydrate | Bael fruit ash (BFA) | Water | Room temperature stirring | 30 min | 86–94% | Shinde et al.81 |
Bio-catalysis is an entirely eco-friendly method that eliminates reliance on metal catalysts and emphasizes natural products. Yet, further research is necessary to enhance reaction speed through a comprehensive understanding of catalytic efficiency and mechanisms.
4 Green solvents
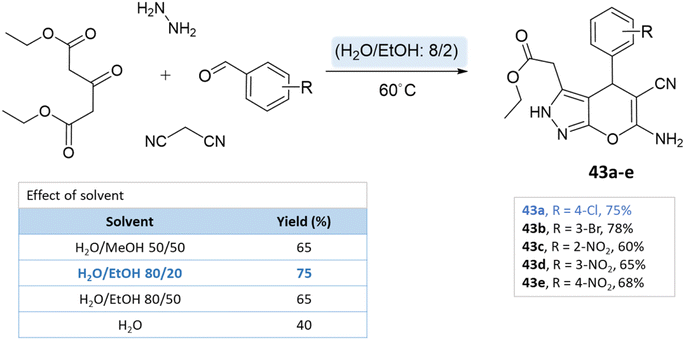
Green solvents are crucial in organic synthesis due to their reduced environmental impact and health hazards compared to conventional solvents. Their use aligns with eco-friendly principles, driving cleaner and more sustainable organic synthetic practices.82 For instance, water, supercritical CO2, and ionic liquids have gained prominence. They offer advantages like improved atom economy, lower toxicity, and easier product separation.834.1 Water
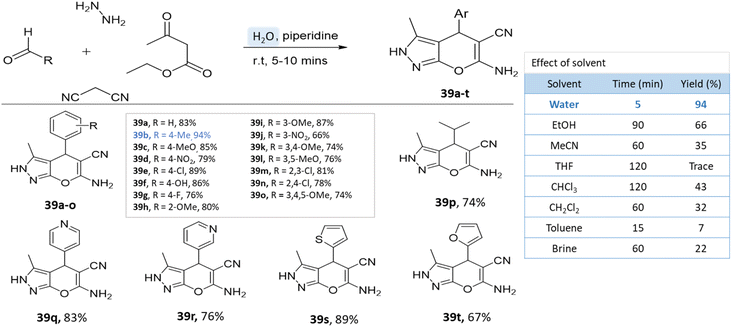
Water has emerged as a prominent green solvent in organic synthesis due to its abundance, low cost, and environmental benignity. Water's characteristics, including its high polarity and unique hydrogen bonding, facilitate various reactions. Advantages include improved safety, minimized waste, and facile product isolation. It promotes eco-friendly synthesis for heterocyclic compounds such as pyrano-pyrazoles by enabling milder conditions and reducing the need for toxic organic solvents, thereby aligning with green chemistry principles.84,85A series of pyranopyrazole derivatives were synthesized in an aqueous medium by Vasuki and Kumaravel86 through the multi-component strategy. At room temperature, the reaction was carried out between ethyl acetoacetate, hydrazine hydrate, malononitrile, and various benzaldehyde in the presence of piperidine. Water proved to be the most effective solvent when compared to typical organic solvents (Scheme 39).
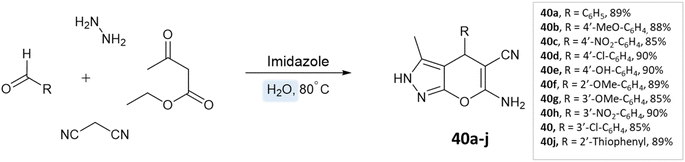
Siddekha et al.87 investigated the influence of different solvents dichloromethane (DCM), acetonitrile (CH3CN), ethanol, and water, for a model reaction of pyrano[2,3-c]pyrazole synthesis, employing a small amount of imidazole as an organocatalyst. Among the solvents tested, water demonstrated the highest yields within a relatively short reaction time of 20–30 min. Consequently, the aromatic aldehydes, malononitrile, hydrazine hydrate, ethyl acetoacetate, and imidazole were dissolved in water and the reaction mixture was heated on a preheated hot plate at 80 °C for 20–30 min (Scheme 40).
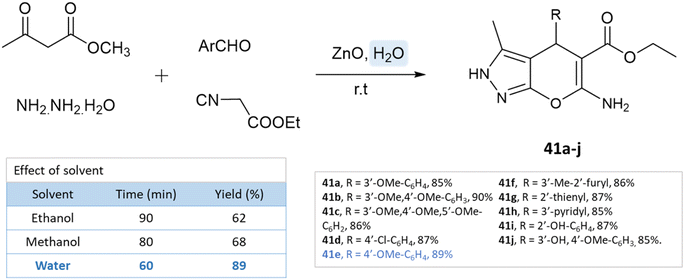
Using ZnO nanoparticles at room temperature, Sachdeva and Saroj88 investigated the solvent effect for the synthesis of pyrano[2,3-c]pyrazole-5-carboxylate derivatives. While yields in ethanol and methanol are poor, the best results are obtained with water (Scheme 41).
A study conducted by Mingshu Wu et al.89 presented a facile approach for the 6-amino-3-methyl-4-aryl-(1-phenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile synthesis, utilizing water as the solvent. An aqueous solution of hydrazine hydrate or phenylhydrazine with ethyl acetoacetate was added with CTACl (cetyl-trimethyl-ammonium chloride), malononitrile, and substituted aldehyde (Scheme 42).
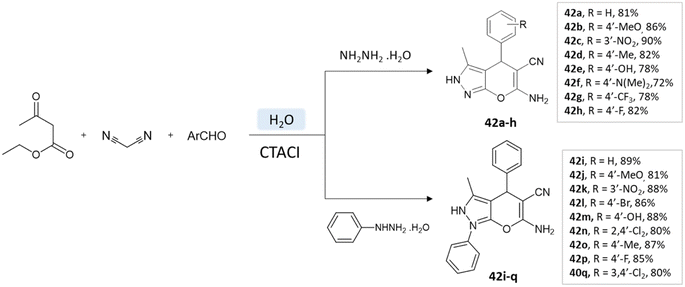 | ||
| Scheme 42 Pyrano[2,3-c] pyrazole synthesis in an aqueous medium using cetyltrimethyl-ammonium chloride. | ||
Majid Koohshari et al.90 have outlined a method for synthesizing pyrano[2,3-c]pyrazoles where dialkyl 3-oxopentanedioate, aromatic aldehydes containing electron-withdrawing groups were taken, along with hydrazine hydrate and malononitrile. The reaction takes place in a water/ethanol mixture without the need for a catalyst (Scheme 43). This method was effectively used to produce the spiro-pyrano[2,3-c] pyrazoles from carbonyl compounds, isatin derivatives, and acenaphthenequinone (Scheme 44).
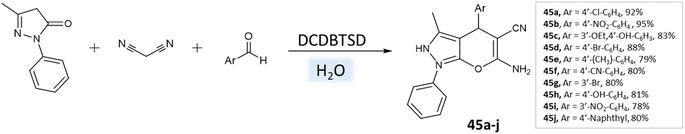
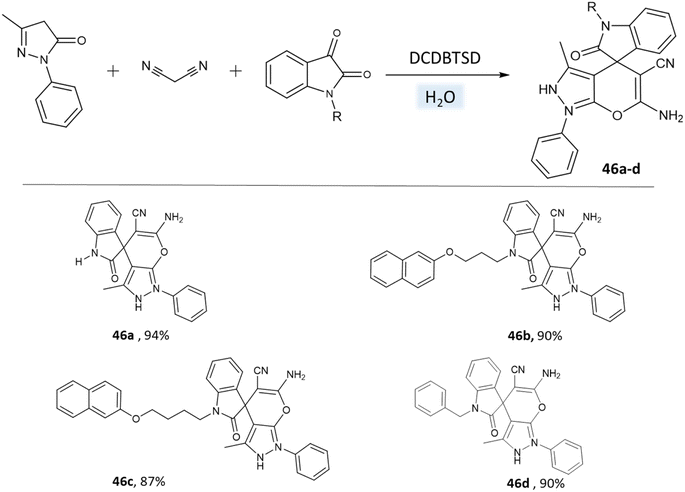
Khazaei et al.91 employed DCDBTSD (N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[1,2,4]thiadiazine-7-sulfonamide-1,1-dioxide) catalyst to facilitate the synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles and spiro-pyrano[2,3-c]pyrazoles in an aqueous medium via multicomponent reaction (Schemes 45 and 46). This method was also applied for the preparation of 4H-pyran, pyrazolo[1,2-b]phthalazine, and spirooxindoles, as detailed in the report.
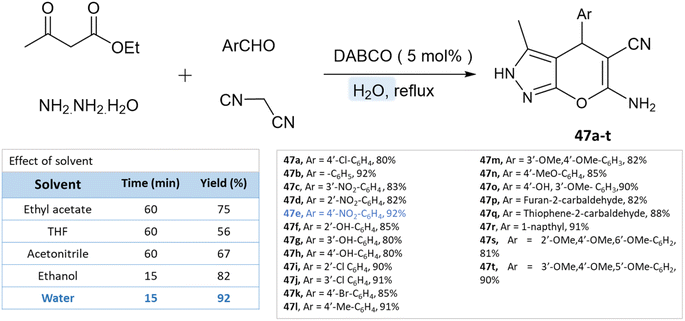
As reported by Waghmare et al.92 dihydropyrano[2,3-c]pyrazoles were synthesized in an aqueous medium with DABCO (1,4-diazabicyclo[2.2.2]octane) via a four-component reaction of ethylacetoacetate, hydrazine hydrate, malononitrile, and various aldehydes. Various solvents were studied for the probe reaction, including ethyl acetoacetate, tetrahydrofuran, acetonitrile, ethanol, and water. Water produced the highest yield when compared to organic solvents (Scheme 47).
Under the ultrasound irradiation method, Priya M. Khandare et al.93 synthesized pyranopyrazoles in an aqueous medium using lanthanum(III) nitrate as a catalyst. The reaction conditions were optimized by performing the model reaction of 4-hydroxybenzaldehyde, ethyl acetoacetate, hydrazine hydrate, and lanthanum(III) nitrate under different conditions. Using the ultrasonication method and water as a solvent, high yields were achieved in a short time (Scheme 48).
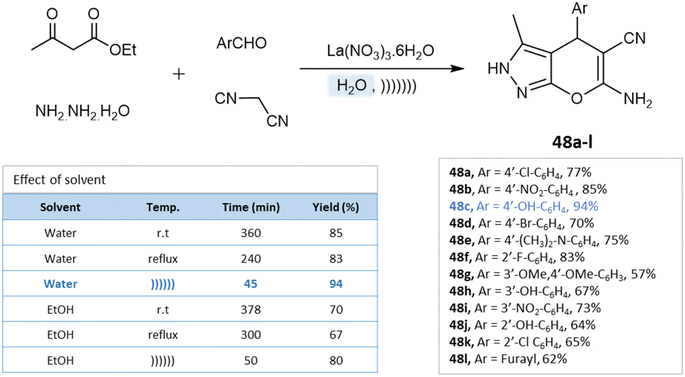 | ||
| Scheme 48 Synthesis of pyrano[2,3-c]pyrazole derivatives in aqueous medium using lanthanum(III) nitrate. | ||
A series of thiadiazole-pyranopyrazole derivatives was amalgamated by M. Reddy et al.94 via the multicomponent reaction of 5-methyl-1,3,4-thiadiazole-2-thiol, hydrazine hydrate, ethyl 4-chloro-3-oxobutanoate, malononitrile, and aryl aldehydes using K10 clay as a green catalyst and ethanol–water as solvent media (Scheme 49). Furthermore, the reaction was also conducted in solvent-free media, leading to low yields with impurities. Solvent-mediated reactions, however, resulted in quantitative yields with no byproducts or impurities.
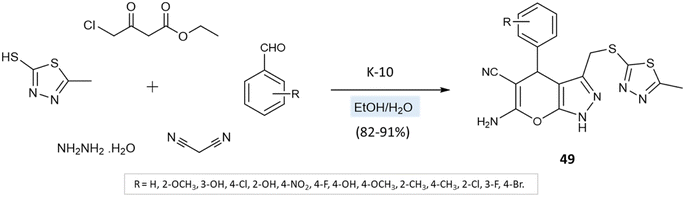 | ||
| Scheme 49 Synthesis of thiadiazole-pyranopyrazole derivatives in water/ethanol medium using K-10 clay. | ||
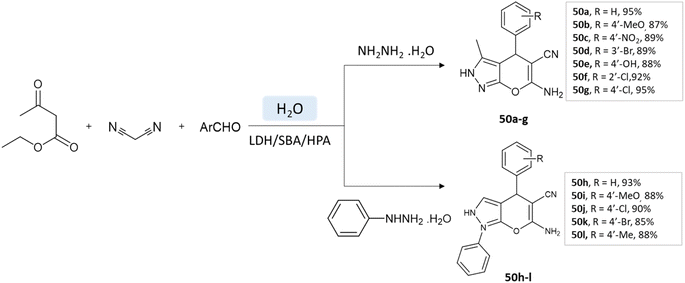
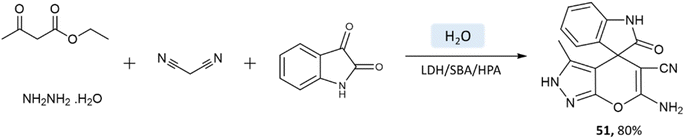
Samahe Sadjadi et al.95 examined the catalytic activity of a ternary hybrid catalyst including HPA (heteropolyacids), LDH (layered double hydroxides), and SBA-15 (mesoporous silica) for the development of pyranopyrazole and spiro-pyranopyrazoles derivatives in aqueous media. A mixture of hydrazine hydrate or phenylhydrazine, ethyl acetoacetate, malononitrile, and aldehyde in the presence of LDH/SBA/HPA was refluxed in water for 15 min (Schemes 50 and 51).
Under reflux conditions with water/ethanol, dihydropyrano[2,3-c]pyrazoles were synthesized from hydrazine hydrate, ethyl acetoacetate, malononitrile, and benzaldehyde by Babaei and Mirjalili96 using nano-Al2O3/BF3/Fe3O4 as catalysts (Scheme 52).
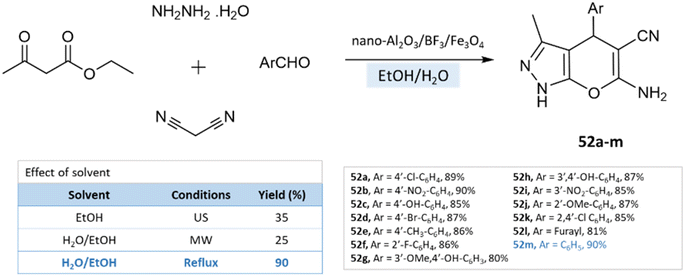 | ||
| Scheme 52 Synthesis of pyrano[2,3-c]pyrazole derivatives in the water–ethanol medium using nano-Al2O3/BF3/Fe3O4. | ||
G. Kargar and coworkers97 synthesized pyranopyrazole derivatives in an aqueous medium using a multi-core catalyst Fe3O4@NFC@Co(II) from ethylacetoacetate, hydrazine hydrate, aldehyde, malononitrile, and Fe3O4@NFC@Co(II) with vigorous stirring at 50 °C, resulting in excellent yields of the pyranopyrazoles in a short time (Scheme 53).
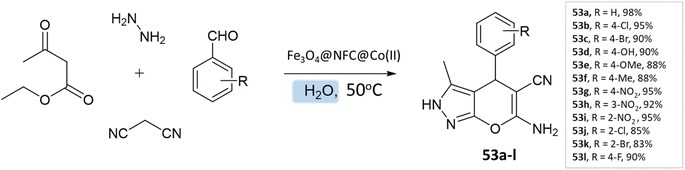 | ||
| Scheme 53 Synthesis of pyrano[2,3-c]pyrazole derivatives in aqueous medium using Fe3O4@NFC@Co(II) as the catalyst. | ||
Fatemeh Mir et al.98 reported the synthesis of dihydropyrano[2,3-c]pyrazole derivatives using a reusable Fe3O4@THAM-piperazine catalyst. The reaction is carried out in an ethanol/water medium, where initially 3-methyl-2-pyrazoline-5-one was precipitated using the reaction between hydrazine hydrate, and ethyl acetoacetate at room temperature, to which then aromatic aldehydes, malononitrile, Fe3O4@THAM-piperazine were added to the reaction mixture and stirred for the suitable time at 60 °C (Scheme 54).
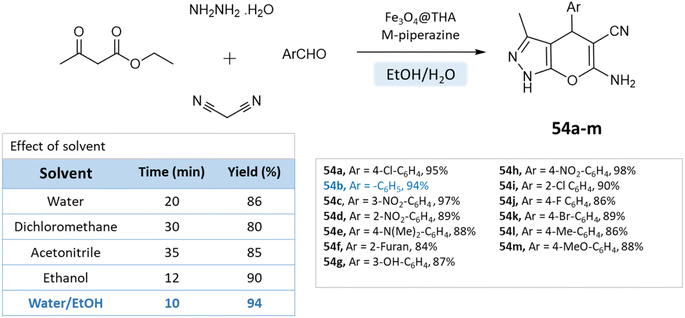 | ||
| Scheme 54 Synthesis of pyrano[2,3-c]pyrazole derivatives in water–ethanol medium using Fe3O4@THAM-piperazine as the catalyst. | ||
Given its safety, lack of toxicity, and natural abundance, water as a solvent offers a promising direction for pyrano-pyrazole synthesis. Nonetheless, careful selection of reaction parameters, including temperature, time, and catalyst, is crucial to optimize the yields (Table 6). Variability in mechanisms must be considered and managed accordingly.
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Pyrano-[2,3-c]-pyrazoles | Four-component system: hydrazine hydrate, ethyl acetoacetate, malononitrile, and various benzaldehydes | Piperidine | Water | Room temperature reflux | 5–10 min | 66–94% | G. Vasuki and Kumaravel86 |
| Pyrano-[2,3-c]-pyrazoles | Four-component system: aromatic aldehydes, malononitrile, ethyl acetoacetate, hydrazine hydrate | Imidazole | Water | Reflux, heating (80 °C) | 20–30 min | 85–90% | Siddekha et al.87 |
| Pyrano[2,3-c]pyrazole-5-carboxylate derivatives | Four-component system: aromatic aldehyde, ethyl 3-cyanopropanoate, ethyl acetoacetate, hydrazine hydrate | ZnO NPs | Water | Room temperature stirring | 30–60 min | 85–90% | Sachdeva and Saroj88 |
| 6-Amino-3-methyl-4-aryl-(1-phenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile | Four-component system: aldehyde, malononitrile, phenylhydrazine or hydrazine hydrate, and ethyl acetoacetate | CTACl | Water | Reflux, heating (90 °C) | 4 h | 72–90% | Mingshu Wu et al.89 |
| Pyrano[2,3-c]pyrazoles | Four-component system: dialkyl 3-oxopentanedioate, aromatic aldehydes, hydrazine, and malononitrile | Catalyst free | Water/EtOH | Reflux, heating (60 °C) | 12 h | 60–78% | Koohshari et al.90 |
| 1,4-Dihydropyrano[2,3-c]pyrazoles and spiro-pyrano[2,3-c]pyrazoles | Four-component system: aromatic aldehydes, malononitrile, ethyl acetoacetate, hydrazine hydrate | DCDBTSD | Water | Reflux, heating (80 °C) | 20–45 min | 79–95% | Khazaei et al.91 |
| Dihydropyrano[2,3-c]pyrazoles | Four-component system: ethylacetoacetate, hydrazine hydrate, malononitrile and various aldehydes | DABCO | Water | Room temperature reflux | 15 min | 80–92% | Waghmare et al.92 |
| Pyrano[2,3-c]pyrazoles | Four-component system: 4-hydroxybenzaldehyde, ethyl acetoacetate, hydrazine hydrate, malononitrile | Lanthanum(III) nitrate | Water | Ultrasound | 45–60 min | 62–94% | M. Khandare et al.93 |
| Thiadiazole-pyranopyrazoles | Four-component system: 5-methyl-1,3,4-thiadiazole-2-thiol, ethyl 4-chloro-3-oxo butanoate, hydrazine hydrate, malononitrile, and aryl aldehydes | K10 clay | Water/EtOH | Heating (65–70 °C) | 5 h | 82–91% | M. Reddy et al.94 |
| Pyrano[2,3-c]pyrazole and spiro-pyrano[2,3-c]pyrazoles | Four-component system: hydrazine hydrate or phenylhydrazine, ethyl acetoacetate, aldehyde, and malononitrile | Ternary hybrid catalyst including HPA, LDH, and SBA-15 | Water | Reflux | 15 min | 85–95% | Samahe Sadjadi et al.95 |
| Dihydropyrano[2,3-c]pyrazoles | Four-component system: hydrazine hydrate, ethyl acetoacetate, malononitrile, and benzaldehyde | Nano-Al2O3/BF3/Fe3O4 | Water/EtOH | Reflux | 25–40 min | 80–90% | Babaei and Mirjalili96 |
| Pyrano[2,3-c]pyrazoles | Four-component system: hydrazine hydrate, ethyl acetoacetate, aldehyde, malononitrile | Fe3O4@NFC@Co(II) | Water | Reflux, heating (50 °C) | 10–20 min | 83–98% | G. Kargar et al.97 |
| Pyrano[2,3-c]pyrazoles | Four-component system: hydrazine hydrate, ethyl acetoacetate, aldehyde, malononitrile | Fe3O4@THAM-piperazine | Water/EtOH | Heating (60 °C) | 10–25 min | 84–97% | Fatemeh Mir et al.98 |
4.2 Solvent-free synthesis
Solvent-free organic synthesis is an environmentally friendly approach that aims to reduce its ecological footprint by shunning conventional solvents and minimizing waste generation. This method boasts several notable characteristics, including shorter reaction times and simplified purification processes. These attributes confer several advantages, such as heightened safety, increased yield, and cost-effectiveness. Additionally, there is no need to evaporate solvents or heat them to overcome their boiling points.99–101An effective solvent-free four-component synthesis of functionalized pyranopyrazoles was developed by Kanagaraj et al.102 using per-6-amino-β-cyclodextrin as the catalyst. A small amount of per-6-ABCD (0.008 mmol) combined with hydrazine hydrate, ethyl acetoacetate, aldehyde/ketone, and malononitrile yields quantitative yields of pyranopyrazoles in about a minute under solvent-free conditions (Scheme 55). This solid base catalyst can be reused six times without losing any of its catalytic activity.
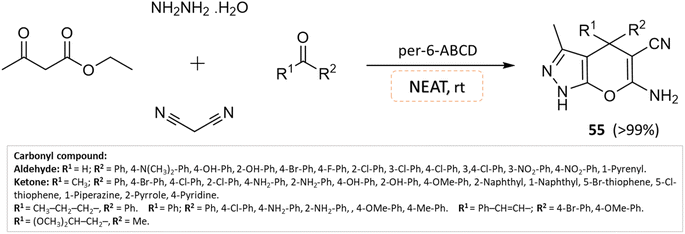 | ||
| Scheme 55 Synthesis of pyrano[2,3-c]pyrazole derivatives in solvent-free conditions using per-6-ABCD as the catalyst. | ||
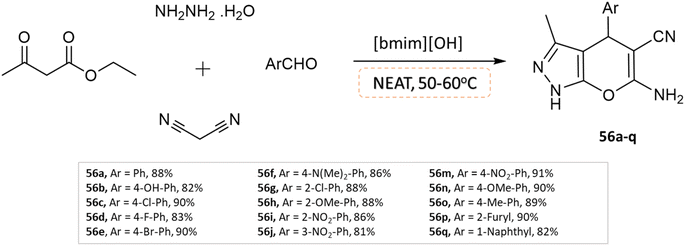
Khurana and Chaudhary103 developed 4H-pyrano[2,3-c]pyrazoles using [bmim]OH as a task-specific ionic liquid via four-component condensation of aldehydes, malononitrile, ethyl acetoacetate, and hydrazine monohydrate without any use of a solvent (Scheme 56).
J. Ebrahimi et al.104 reported the synthesis of biologically active pyranopyrazoles under a solvent-free environment via condensation of various aromatic aldehydes, ethyl acetoacetate, hydrazine hydrate, malononitrile in the presence of [(CH2)4SO3HMIM][HSO4], as an ionic liquid and catalyst (Scheme 57).
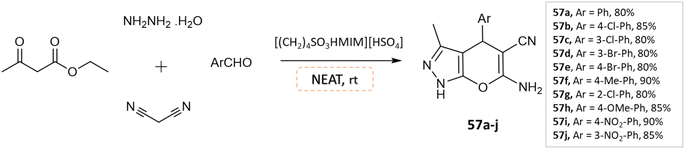 | ||
| Scheme 57 Synthesis of pyrano[2,3-c]pyrazole derivatives in solvent-free conditions using [(CH2)4SO3HMIM][HSO4]. | ||
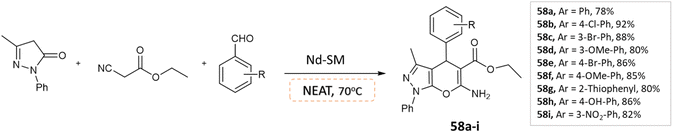
Abdullah Rather et al.105 reported a three-component one-pot synthesis of pyranopyrazole moieties via condensation of ethyl cyanoacetate, aldehyde, and pyrazolone under solvent-free conditions using a recyclable heterogeneous catalyst NdSM (Scheme 58). The study reports the application and synthesis of Nd-Salen immobilized mesoporous silica (NdSM) as a catalyst.
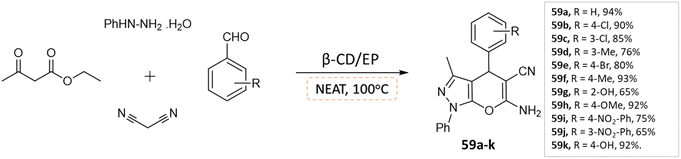
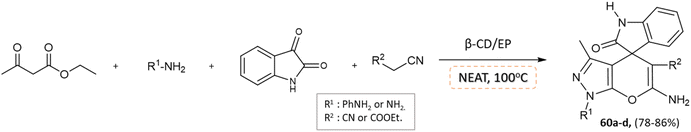
J. Nasab and colleagues106 developed spiro[indoline-3,4′-pyrano(2,3-c)pyrazole] and pyranopyrazole derivatives using a water-insoluble nanosponge polymer called β-cyclodextrin/epichlorohydrin as a catalyst and a fixed micro-vessel under solvent-free thermal conditions. A mixture of aromatic aldehyde, phenylhydrazine, ethyl acetoacetate, malononitrile, and β-CD/EP was heated at 100 °C without any solvent for the pyranopyrazole synthesis, facilitated by the β-CD/EP catalyst (Scheme 59). For the synthesis of spiro[indoline-3,4′-pyrano(2,3-c)pyrazole] derivatives, a solvent-free reaction was conducted by stirring a mixture of phenylhydrazine, malononitrile, isatin, ethyl acetoacetate, and β-CD/EP at 100 °C. The β-CD/EP catalyst served as a stationary micro-vessel, enabling the reaction to proceed (Scheme 60).
Dadaei and Naeimi107 reported the synthesis of pyrano[2,3-c]pyrazole derivatives at room temperature from ethyl acetoacetate, hydrazine hydrate, different aldehydes, and malononitrile, under a solvent-free environment in the presence of CoCuFe2O4 magnetic nanocrystals as a reusable catalyst (Scheme 61).
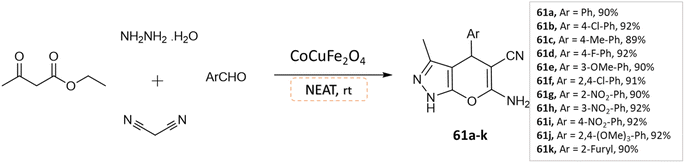 | ||
| Scheme 61 Synthesis of pyrano[2,3-c]pyrazole derivatives in solvent-free conditions using CoCuFe2O4 nanocrystals. | ||
Soleimani and coworkers108 utilized Fe3O4@SiO2@Si(OEt)(CH2)3@melamine@TC@Cu(OAc)2 nanomagnetic catalyst under solvent-free conditions for the synthesis of pyrano[2,3-c]pyrazole derivatives. Reaction of phenylhydrazine, ethyl acetoacetate and benzaldehydes was carried out under the optimized reaction conditions in various solvents, namely ethyl acetate, acetonitrile, water, n-hexane, ethanol, and solvent-free condition. By examining the results of solvent-free conditions, it provides the shortest reaction time and the highest reaction yield. Additionally, the use of the magnetic catalyst significantly reduced the synthesis time of these compounds under solvent-free conditions (Scheme 62).
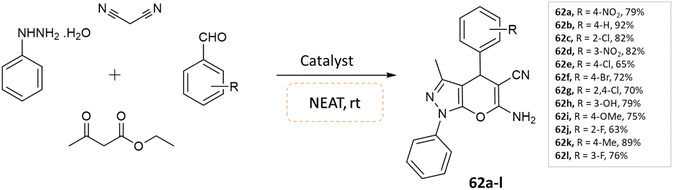 | ||
| Scheme 62 Synthesis of pyrano[2,3-c]pyrazole derivatives in solvent-free conditions using a nanomagnetic catalyst. | ||
S. L. Sangle et al.109 utilized CuSnO3:SiO2 catalyst, synthesized using a hydrothermal method for the synthesis of pyranopyrazoles through a one-pot, four-component reaction involving aldehydes, malononitrile hydrazine hydrate, and ethylacetoacetate under solvent-free conditions (Scheme 63). This method demonstrated high yield and short reaction time, with an economically available catalyst and easy purification. The catalyst also showed potential as an alternative catalyst for various acidic-mediated reactions.
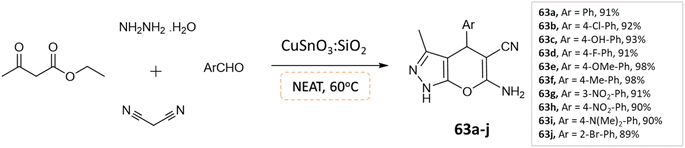 | ||
| Scheme 63 Synthesis of pyrano[2,3-c]pyrazole derivatives in solvent-free conditions using CuSnO3:SiO2 catalyst. | ||
S. Ganesan and P. Suresh110 hydrothermally synthesized nitrogen-doped graphene oxide (NGO) and investigated its application as a solid-base heterogeneous catalyst for pyranopyrazoles synthesis. Malononitrile, ethyl acetoacetate, hydrazine hydrate, and different functional groups of substituted aldehydes were combined in a condensation reaction under solvent-free conditions using a grinding technique, yielding high yields of pyranopyrazoles within 2 min (Scheme 64). Notably, the catalyst material exhibited stability and could be recycled and reused for up to eight consecutive cycles with only a trivial decrease in efficiency.
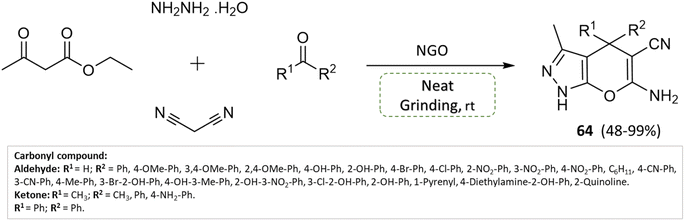 | ||
| Scheme 64 Synthesis of pyrano[2,3-c]pyrazole derivatives through neat grinding using nitrogen-doped graphene oxide. | ||
P. Verma et al.111 devised an eco-friendly approach devoid of metal catalysts, enabling the synthesis of a diverse array of dihydropyrano[2,3-c]pyrazole derivatives. This method involves 3-methyl pyrazolone, methyl arenes, malononitrile, and leverages urea hydrogen peroxide within a multicomponent reaction. Notably, this reaction is carried out under the solvent-free grinding method at ambient temperature (Scheme 65).
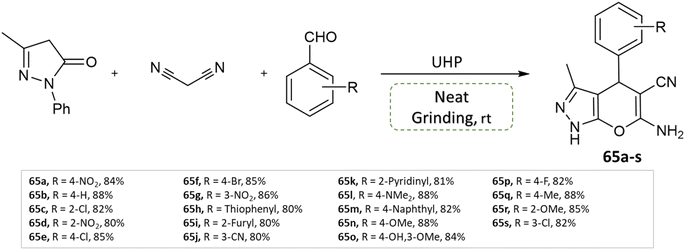 | ||
| Scheme 65 Synthesis of pyrano[2,3-c]pyrazole derivatives through neat grinding using urea hydrogen peroxide. | ||
V. Sapkal et al.112 reported an efficient, green, and facile multi-component one-pot synthesis of pyrano[2,3-c] pyrazoles with various aryl aldehyde, malononitrile, ethyl acetoacetate, hydrazine hydrate under solvent-free grinding condition using ionic liquid (NMPyTs) as a catalyst (Scheme 66). A notable advantage of this protocol is its simplicity, solvent-free approach, easy workup, high yield, neat and clean synthesis (Table 7).
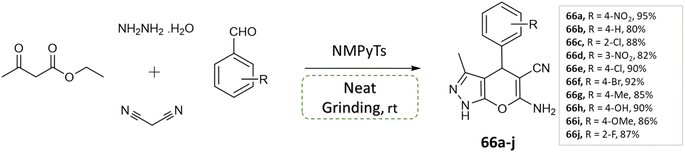 | ||
| Scheme 66 Synthesis of pyrano[2,3-c]pyrazole derivatives through neat grinding using ionic liquid (NMPyTs). | ||
| Product | Reactants | Catalyst | Solvent | Method employed | Reaction time | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Pyrano[2,3-c]pyrazoles | Four-component system: hydrazine hydrate, ethyl acetoacetate, aldehyde/ketone, and malononitrile | Per-6-amino-β-cyclodextrin | Solvent-free | Room temperature mixing | 1–2 min | 90–99% | Kanagaraj et al.102 |
| 4H-Pyrano[2,3-c]pyrazoles | Four-component system: aldehydes, malononitrile, ethyl acetoacetate, and hydrazine monohydrate | [bmim]OH ionic liquid | Solvent-free | Heating (50–60 °C) | 5–10 min | 81–91% | Khurana and Chaudhary103 |
| Biologically active substituted pyranopyrazoles | Four-component system: aromatic aldehydes, ethyl acetoacetate, hydrazine hydrate, malononitrile | [(CH2)4SO3HMIM][HSO4] | Solvent-free | Room temperature stirring | 30 min | 80–90% | J. Ebrahimi et al.104 |
| Pyrano[2,3-c]pyrazoles | Three-component system: aldehyde, ethyl cyanoacetate, and pyrazolone | Nd-Salen immobilized mesoporous silica (NdSM) | Solvent-free | Heating (70 °C) | 10–20 min | 78–92% | A. Rather et al.105 |
| Pyranopyrazole derivatives | Four-component system: aromatic aldehyde, phenylhydrazine, ethyl acetoacetate, malononitrile | β-Cyclodextrin/epichlorohydrin (β-CD/EP) | Solvent-free | Heating (100 °C) | 20–60 min | 65–94% | J. Nasab et al.106 |
| Pyrano[2,3-c]pyrazoles | Four-component system: ethyl acetoacetate, hydrazine hydrate, malononitrile, and aryl aldehydes | CoCuFe2O4 magnetic nanocrystals | Solvent-free | Room temperature stirring | 40–45 min | 89–92% | Dadaei and Naeimi107 |
| Pyrano[2,3-c]pyrazoles | Four-component system: malononitrile, ethyl acetoacetate, phenyl hydrazine hydrate, and substituted aldehydes | Fe3O4@SiO2@Si(OEt)(CH2)3@melamine@TC@Cu(OAc)2 | Solvent-free | Room temperature stirring | 7–15 min | 63–92% | Soleimani et al.108 |
| Pyrano[2,3-c]pyrazoles | Four-component system: malononitrile, ethyl acetoacetate, hydrazine hydrate, and substituted aldehydes | CuSnO3:SiO2 | Solvent-free | Heating (60 °C) | 10 min | 90–98% | S. L. Sangle et al.109 |
| Pyrano[2,3-c]pyrazoles | Four-component system: malononitrile, ethyl acetoacetate, hydrazine hydrate, and substituted aldehydes | Nitrogen-doped graphene oxide (NGO) | Solvent-free (physical grinding) | Room temperature mixing | 2 min | 48–99% | S. Ganesan et al.110 |
| Dihydropyrano[2,3-c]pyrazoles | Three-component system: methyl arenes, 3-methyl pyrazolone, and malononitrile | Urea hydrogen peroxide | Solvent-free (physical grinding) | Room temperature mixing | 15–25 min | 80–88% | P. Verma et al.111 |
| Pyrano[2,3-c]pyrazoles | Four-component system: malononitrile, ethyl acetoacetate, hydrazine hydrate, and substituted aldehydes | NMPyTs (ionic liquid; boiled at 120 °C) | Solvent-free (physical grinding) | Room temperature mixing | 10 min | 80–95% | Amol V. Sapkal et al.112 |
The solvent-free approach offers numerous benefits, including cost-effectiveness, ease of purification, and excellent yields. It should be explored to optimize reaction time and other factors. Moreover, combining diverse green methodologies might enhance outcomes.
5 Conclusion
This review integrates several green methodologies that demonstrate their potential in the creation of structurally diverse and biologically relevant pyrano[2,3-c]pyrazole and spiro-pyrano[2,3-c]pyrazole derivatives. The article has analyzed both the notable benefits and constraints of these synthetic approaches to highlight forthcoming research directions, which prioritize safer reaction conditions, enhanced environmental factors, increased yields alongside improved selectivity, and the elimination of hazardous precursors, among other factors.Techniques such as microwave heating, concentrated solar radiation, and ultrasound irradiation have emerged as rapid and energy-efficient alternatives to conventional heating. However, it is imperative that future investigations carefully consider scalability and reaction conditions to ensure practicality and effectiveness.
Catalytic strategies, including nano-catalysis, organo-catalysis, and bio-catalysis, have demonstrated unique advantages such as accelerated reactions and improved selectivity, all while maintaining an eco-friendly profile. Nevertheless, sustainable catalyst synthesis and a deeper understanding of their mechanisms are essential to maximize their efficiency in pyranopyrazole synthesis.
The pivotal role of water as a green solvent cannot be overstated, as it enables milder reaction conditions and reduces reliance on toxic organic solvents. Additionally, solvent-free synthesis aligns seamlessly with green principles, offering better results and cost-effective reactions. Exploring emerging green solvents with a keen focus on reaction parameters holds great potential for the synthesis of novel pyranopyrazole derivatives.
While significant strides have been made in the development of green multicomponent reactions for pyranopyrazole synthesis, there are still ample opportunities for further exploration and advancements. We hope that this comprehensive review article not only serves as a catalyst for further research but also inspires researchers to adopt these green approaches, leading to cleaner and more sustainable processes.
Author contributions
Afrisham Ahmad: writing – original draft, conceptualization, Sithara Rao: formal analysis, validation, Nitinkumar S. Shetty: supervision, visualization.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We want to express our profound gratitude to the Manipal Institute of Technology, MAHE.References
- R. S. Varma, ACS Sustain. Chem. Eng., 2016, 4, 5866–5878 CrossRef CAS PubMed.
- O. V. Kharissova, B. I. Kharisov, C. M. O. González, Y. P. Méndez and I. López, R. Soc. Open Sci., 2019, 6, 191378 CrossRef PubMed.
- E. Van der Eycken and U. K. Sharma, Multicomponent Reactions towards Heterocycles, Wiley, 2022 Search PubMed.
- G. M. Reddy, A. K. Kumari, V. H. Reddy and J. R. Garcia, Bioorg. Chem., 2021, 41(2), 223–291 Search PubMed.
- H. Kashtoh, M. T. Muhammad, J. J. A. Khan, S. Rasheed, A. Khan, S. Perveen, K. Javaid, A. Wahab, K. M. Khan and M. I. Choudhary, Bioorg. Chem., 2016, 65, 61–72 CrossRef CAS PubMed.
- D. Becerra, R. Abonia and J.-C. Castillo, Molecules, 2022, 27, 4723 CrossRef CAS PubMed.
- M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295–4298 CrossRef CAS PubMed.
- S. R. Mandha, S. Siliveri, M. Alla, V. R. Bommena, M. R. Bommineni and S. Balasubramanian, Bioorg. Med. Chem. Lett., 2012, 22, 5272–5278 CrossRef CAS PubMed.
- S. A. Patil, J. Wang, X. S. Li, J. Chen, T. S. Jones, A. Hosni-Ahmed, R. Patil, W. L. Seibel, W. Li and D. D. Miller, Bioorg. Med. Chem. Lett., 2012, 22, 4458–4461 CrossRef CAS PubMed.
- L.-J. Huang and H. Nakamuraf Sheng-Chu Kuo, J. Med. Chem., 1984, 27(4), 539–544 CrossRef PubMed.
- P. W. Smith, S. L. Sollis, P. D. Howes, P. C. Cherry, I. D. Starkey, K. N. Cobley, H. Weston, J. Scicinski, A. Merritt, A. Whittington, P. Wyatt, N. Taylor, D. Green, R. Bethell, S. Madar, R. J. Fenton, P. J. Morley, T. Pateman and A. Beresford, Dihydropyrancarboxamides Related to Zanamivir: A New Series of Inhibitors of Influenza Virus Sialidases. 1. Discovery, Synthesis, Biological Activity, and Structure–Activity Relationships of 4-Guanidino-and 4-Amino-4H-pyran-6-carboxamides, 1998 Search PubMed.
- N. Foloppe, L. M. Fisher, R. Howes, A. Potter, A. G. S. Robertson and A. E. Surgenor, Bioorg. Med. Chem., 2006, 14, 4792–4802 CrossRef CAS PubMed.
- K. Qvortrup, J. F. Jensen, M. S. Sørensen, I. Kouskoumvekaki, R. K. Petersen, O. Taboureau, K. Kristiansen and T. E. Nielsen, PLoS One, 2017, 12(2), e0162642 CrossRef PubMed.
- M. Mamaghani and R. Hossein Nia, Polycyclic Aromat. Compd., 2021, 41, 223–291 CrossRef CAS.
- S. V. H. S. Bhaskaruni, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Arabian J. Chem., 2020, 13, 1142–1178 CrossRef CAS.
- R. S. Varma, ACS Sustain. Chem. Eng., 2016, 4, 5866–5878 CrossRef CAS PubMed.
- A. Kate, L. K. Sahu, J. Pandey, M. Mishra and P. K. Sharma, Curr. Res. Green Sustainable Chem., 2022, 5, 100248 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743–775 RSC.
- D. K. Romney, F. H. Arnold, B. H. Lipshutz and C. J. Li, J. Org. Chem., 2018, 83, 7319–7322 CrossRef CAS PubMed.
- A. H. M. Elwahy and M. R. Shaaban, RSC Adv., 2015, 5, 75659–75710 RSC.
- H. Hegde and N. S. Shetty, Chem. Heterocycl. Compd., 2017, 53, 883–886 CrossRef CAS.
- A. Bhope, A. Asnani, D. Chaple, V. Nimbekar and P. Badne, World J. Pharmaceut. Res., 2022, 11(2), 627–647 CAS.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- Y. Gu, Green Chem., 2012, 14, 2091–2128 RSC.
- N. Kerru, L. Gummidi, S. Maddila and S. B. Jonnalagadda, Sustainable Chem. Pharm., 2020, 18, 100316 CrossRef.
- N. Kerru, S. V. H. S. Bhaskaruni, L. Gummidi, S. N. Maddila, S. Rana, P. Singh and S. B. Jonnalagadda, Appl. Organomet. Chem., 2019, 33(5), 4722 CrossRef.
- B. Ardiansah, Int. J. ChemTech Res., 2019, 12(5), 273–280 CAS.
- S. Sikandar and A. F. Zahoor, J. Heterocycl. Chem., 2021, 58, 685–705 CrossRef CAS.
- R. B. Nasir Baiga and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC.
- R. S. Varma, Green Chem., 2014, 16, 2027–2041 RSC.
- A. Singh Grewal, Int. Res. J. Pharm. Appl. Sci., 2013, 3(5), 278–285 Search PubMed.
- C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC.
- H. G. Kathrotiya, R. G. Patel and M. P. Patel, J. Serb. Chem. Soc., 2012, 77, 983–991 CrossRef CAS.
- N. J. Parmar, H. A. Barad, B. R. Pansuriya and N. P. Talpada, RSC Adv., 2013, 3, 8064–8070 RSC.
- P. Shukla, A. Sharma, S. Anthal and R. Kant, Bull. Mater. Sci., 2015, 38, 1119–1127 CrossRef CAS.
- B. D. Rupnar, V. P. Pagore, S. U. Tekale, S. U. Shisodia and R. P. Pawar, Chem. Sin., 2017, 8(2), 229–234 CAS.
- M. S. Vasava, M. N. Bhoi, S. K. Rathwa, S. S. Shetty, R. D. Patel, D. P. Rajani, S. D. Rajani, A. Patel, H. A. Pandya and H. D. Patel, J. Mol. Struct., 2019, 1181, 383–402 CrossRef CAS.
- G. N. Yallappa, N. Dasappa, U. Chandrashekhar and G. L. Aruna, Letters in Applied NanoBioScience, 2022, 11, 3441–3448 Search PubMed.
- F. Mohamadpour, Sci. Rep., 2023, 13, 11485 CrossRef CAS PubMed.
- W. T. Xie, Y. J. Dai, R. Z. Wang and K. Sumathy, Renewable Sustainable Energy Rev., 2011, 15, 2588–2606 CrossRef.
- Y. U. Gadkari, N. T. Hatvate and V. N. Telvekar, Res. Chem. Intermed., 2021, 47, 4245–4255 CrossRef CAS.
- S. Puri, B. Kaur, A. Parmar and H. Kumar, Curr. Org. Chem., 2013, 17, 1790–1828 CrossRef CAS.
- T. J. Mason, Chem. Soc. Rev., 1997, 26, 443–451 RSC.
- K. Ablajan, W. Liju, Y. Kelimu and F. Jun, Mol. Diversity, 2013, 17, 693–700 CrossRef CAS PubMed.
- A. Dandia, V. Parewa, A. K. Jain and K. S. Rathore, Green Chem., 2011, 13, 2135–2145 RSC.
- N. G. Shabalala, R. Pagadala and S. B. Jonnalagadda, Ultrason. Sonochem., 2015, 27, 423–429 CrossRef CAS PubMed.
- S. Maddila, S. Gorle, S. Shabalala, O. Oyetade, S. N. Maddila, P. Lavanya and S. B. Jonnalagadda, Arabian J. Chem., 2019, 12, 671–679 CrossRef CAS.
- R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CrossRef CAS.
- P. T. Anastas, M. M. Kirchhoff and T. C. Williamson, Appl. Catal., A, 2001, 221, 3–13 CrossRef CAS.
- V. Maciulis, A. Ramanaviciene and I. Plikusiene, Nanomaterials, 2022, 12, 4413 CrossRef CAS PubMed.
- R. K. Ganta, N. Kerru, S. Maddila and S. B. Jonnalagadda, Molecules, 2021, 26 Search PubMed.
- R. S. Varma, Sustainable Chem. Processes, 2014, 2, 11 CrossRef.
- S. U. Tekale, S. S. Kauthale, K. M. Jadhav and R. P. Pawar, J. Chem., 2013, 2013, 1–8 CrossRef.
- A. Azarifar, R. Nejat-Yami, M. Al Kobaisi and D. Azarifar, J. Iran. Chem. Soc., 2013, 10, 439–446 CrossRef CAS.
- M. I. A. Abdel Maksoud, A. M. Elgarahy, C. Farrell, A. H. Al-Muhtaseb, D. W. Rooney and A. I. Osman, Coord. Chem. Rev., 2020, 403, 213096 CrossRef.
- L. Xu, S. Zhang, W. Li and Z. Zhang, Chem.–Eur. J., 2021, 27, 5483–5491 CrossRef CAS PubMed.
- M. A. E. A. A. A. El-Remaily, Tetrahedron, 2014, 70, 2971–2975 CrossRef CAS.
- K. Pradhan, S. Paul and A. R. Das, Catal. Sci. Technol., 2014, 4, 822–831 RSC.
- E. Soleimani, M. Jafarzadeh, P. Norouzi, J. Dayou, C. S. Sipaut, R. F. Mansa and P. Saei, J. Chin. Chem. Soc., 2015, 62, 1155–1162 CrossRef CAS.
- M. Beiranvand and D. Habibi, Sci. Rep., 2022, 12(1), 14347 CrossRef CAS PubMed.
- S. N. Maddila, S. Maddila, W. E. van Zyl and S. B. Jonnalagadda, Res. Chem. Intermed., 2017, 43, 4313–4325 CrossRef CAS.
- K. G. Patel, N. M. Misra, R. H. Vekariya and R. R. Shettigar, Res. Chem. Intermed., 2018, 44, 289–304 CrossRef CAS.
- S. Shahbazi, M. A. Ghasemzadeh, P. Shakib, M. R. Zolfaghari and M. Bahmani, Polyhedron, 2019, 170, 172–179 CrossRef CAS.
- M. Mishra, A. Nizam, K. J. Jomon and K. Tadaparthi, Russ. J. Org. Chem., 2019, 55, 1925–1928 CrossRef CAS.
- E. Sedighinia, R. Badri and A. R. Kiasat, Russ. J. Org. Chem., 2019, 55, 1755–1763 CrossRef CAS.
- P. Chhattise, S. Saleh, V. Pandit, S. Arbuj and V. Chabukswar, Mater. Adv., 2020, 1, 2339–2345 RSC.
- M. Aravind, M. Amalanathan and M. S. M. Mary, SN Appl. Sci., 2021, 3, 409 CrossRef CAS.
- P. K. Dikshit and B. S. Kim, Catalysts, 2022, 13, 27 CrossRef.
- Y. Tao, R. Dong, I. V. Pavlidis, B. Chen and T. Tan, Green Chem., 2016, 18, 1240–1248 RSC.
- G. Fiorani, W. Guo and A. W. Kleij, Green Chem., 2015, 17, 1375–1389 RSC.
- M. B. M. Reddy, V. P. Jayashankara and M. A. Pasha, Synth. Commun., 2010, 40, 2930–2934 CrossRef CAS.
- M. A. Zolfigol, M. Tavasoli, A. R. Moosavi-Zare, P. Moosavi, H. G. Kruger, M. Shiri and V. Khakyzadeh, RSC Adv., 2013, 3, 25681–25685 RSC.
- Y. A. Tayade, S. A. Padvi, Y. B. Wagh and D. S. Dalal, Tetrahedron Lett., 2015, 56, 2441–2447 CrossRef CAS.
- J. P. Sonar, S. D. Pardeshi, S. A. Dokhe, G. M. Bhavar, S. U. Tekale, A. M. Zine and S. N. Thore, Eur. Chem. Bull., 2019, 8(6), 207–211 CrossRef CAS.
- N. D. Jumbam and W. Masamba, Molecules, 2020, 25, 5935 CrossRef CAS PubMed.
- R. Y. Guo, Z. M. An, L. P. Mo, S. T. Yang, H. X. Liu, S. X. Wang and Z. H. Zhang, Tetrahedron, 2013, 69, 9931–9938 CrossRef CAS.
- X. Huang, Z. Li, D. Wang and Y. Li, Chin. J. Catal., 2016, 37, 1461–1467 CrossRef CAS.
- S. S. Ghodke, S. U. Tekale, R. D. Pathrikar, P. M. Khandare, L. Kótai and R. P. Pawar, Eur. Chem. Bull., 2020, 9, 38–42 CrossRef CAS.
- A. Dehghani Tafti, B. B. F. Mirjalili, A. Bamoniri and N. Salehi, BMC Chem., 2021, 15, 6 CrossRef CAS PubMed.
- P. P. Bora, M. Bihani and G. Bez, J. Mol. Catal. B: Enzym., 2013, 92, 24–33 CrossRef CAS.
- S. K. Shinde, M. U. Patil, S. A. Damate and S. P. Patil, Res. Chem. Intermed., 2017, 44, 1775–1795 CrossRef.
- N. Winterton, Clean Technol. Environ. Policy, 2021, 23, 2499–2522 CrossRef PubMed.
- K. Shanab, C. Neudorfer, E. Schirmer and H. Spreitzer, Curr. Org. Chem., 2013, 17, 1179–1187 CrossRef CAS.
- M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- A. V. Dolzhenko and A. V. Dolzhenko, in Green Synthetic Approaches for Biologically Relevant Heterocycles, Elsevier, 2015, pp. 101–139 Search PubMed.
- G. Vasuki and K. Kumaravel, Tetrahedron Lett., 2008, 49, 5636–5638 CrossRef CAS.
- A. Siddekha, A. Nizam and M. A. Pasha, Spectrochim. Acta, Part A, 2011, 81, 431–440 CrossRef CAS PubMed.
- H. Sachdeva and R. Saroj, Sci. World J., 2013, 2013, 1–8 Search PubMed.
- M. Wu, Q. Feng, D. Wan and J. Ma, Synth. Commun., 2013, 43, 1721–1726 CrossRef CAS.
- M. Koohshari, M. Dabiri and P. Salehi, RSC Adv., 2014, 4, 10669–10671 RSC.
- A. Khazaei, M. A. Zolfigol, F. Karimitabar, I. Nikokar and A. R. Moosavi-Zare, RSC Adv., 2015, 5, 71402–71412 RSC.
- A. S. Waghmare and S. S. Pandit, J. Saudi Chem. Soc., 2017, 21, 286–290 CrossRef CAS.
- P. M. Khandare, R. D. Ingale, A. S. Taware, S. U. Shisodia, S. S. Pawar, L. Kotai and R. P. Pawar, European Chemical Bulletin, 2017, 6, 410 CrossRef CAS.
- G. M. Reddy, J. R. Garcia, G. V. Zyryanov, G. Sravya and N. B. Reddy, Bioorg. Chem., 2019, 82, 324–331 CrossRef CAS PubMed.
- S. Sadjadi, M. M. Heravi, V. Zadsirjan and V. Farzaneh, Res. Chem. Intermed., 2018, 44, 6765–6785 CrossRef CAS.
- E. Babaei and B. B. F. Mirjalili, Inorg. Nano-Met. Chem., 2020, 50, 16–21 CrossRef CAS.
- P. G. Kargar, G. Bagherzade and H. Eshghi, RSC Adv., 2020, 10, 37086–37097 RSC.
- F. Mir, N. Hazeri, M. T. Maghsoodlou and M. Lashkari, Polycyclic Aromat. Compd., 2023, 43(6), 5375–5390 CrossRef CAS.
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC.
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547 RSC.
- S. Zangade and P. Patil, Curr. Org. Chem., 2020, 23, 2295–2318 CrossRef.
- K. Kanagaraj and K. Pitchumani, Tetrahedron Lett., 2010, 51, 3312–3316 CrossRef CAS.
- J. M. Khurana and A. Chaudhary, Green Chem. Lett. Rev., 2012, 5, 633–638 CrossRef CAS.
- J. Ebrahimi, A. Mohammadi, V. Pakjoo, E. Bahramzade and A. Habibi, J. Chem. Sci., 2012, 124(5), 1013–1017 CrossRef CAS.
- R. A. Rather and Z. N. Siddiqui, J. Organomet. Chem., 2018, 868, 164–174 CrossRef CAS.
- M. Jafari Nasab, A. R. Kiasat and R. Zarasvandi, React. Kinet., Mech. Catal., 2018, 124, 767–778 CrossRef CAS.
- M. Dadaei and H. Naeimi, Polycyclic Aromat. Compd., 2021, 42, 204–217 CrossRef.
- M. Soleimani, T. Akbarpour and A. Khazaei, Polycyclic Aromat. Compd., 2023 DOI:10.1080/10406638.2023.2169472.
- S. L. Sangle, D. R. Tope, A. V. Borhade and S. S. Ghumare, J. Adv. Sci. Res., 2022, 13, 38–43 CAS.
- N. Saravana Ganesan and P. Suresh, ChemistrySelect, 2020, 5, 4988–4993 CrossRef CAS.
- P. Verma, S. Chauhan, V. Singh, S. Singh and V. Srivastava, Mol. Diversity, 2022, 26, 1769–1777 CrossRef CAS PubMed.
- A. V. Sapkal, D. L. Lingampalle and B. R. Madje, J. Emerging Technol. Innovative Res., 2020, 7(4), 1135–1139 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |