A novel strategy for reversible hydrogen storage in Ca(BH4)2†
Abstract
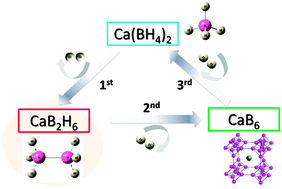
We report that decomposition pathway of Ca(BH4)2 can be efficiently controlled by reaction temperature. That is, it decomposes into CaB6 at a lower temperature range of 320 to 350 °C, but into amorphous boron at 400 to 450 °C. We identified the formation of CaB2H6 as the crucial intermediate step on the way to CaB6 that only forms at 320 to 350 °C.

 Please wait while we load your content...
Please wait while we load your content...