
RSC Advances
Subject area
Organic articles published in the last
6 months

Recent trends in incorporation of CO2 into organosulfur compounds via C–S bond cleavage
Desulfurative functionalization of organosulfur compounds to form various carbon–carbon and carbon–heteroatom bonds has become established as a powerful tool in organic chemistry.

RSC Adv., 2024,14, 15680-15690
https://doi.org/10.1039/D4RA02405B
Easy one-pot synthesis of multifunctionalized indole–pyrrole hybrids as a new class of antileishmanial agents
A chemoselective one-pot synthesis of indole–pyrrole hybrids has been developed. The new hybrids were phenotypically screened for efficacy against L. infantum promastigotes. Compound 3d was the most active with IC50 = 9.6 μM and a selectivity index of 5.

RSC Adv., 2024,14, 15713-15720
https://doi.org/10.1039/D4RA02790F
Iodine-promoted amide formation via oxidative cleavage of indoles: novel quinazoline-4(3H)-one and tryptanthrin syntheses
A highly efficient method for the direct construction of amide bonds via a selective cleavage of C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in indole structures using an iodine-promoted approach was developed.
C bonds in indole structures using an iodine-promoted approach was developed.

RSC Adv., 2024,14, 15597-15603
https://doi.org/10.1039/D4RA01807A
Switchable divergent synthesis of chiral indole derivatives via catalytic asymmetric dearomatization of 2,3-disubstituted indoles
A CPA-catalyzed switchable divergent synthesis of chiral indolenines/indolines via the CADA of 2,3-disubstituted indoles has been described. The post-processing conditions played a key role for the structure switch of the final products.

RSC Adv., 2024,14, 15591-15596
https://doi.org/10.1039/D4RA03231D
Aryl carbonyls and carbinols as proelectrophiles for Friedel–Crafts benzylation and alkylation
Friedel–Crafts benzylation/alkylation using benzylic alcohols and aryl carbonyls as proelectrophiles is achieved using borane-ammonia and TiCl4.

RSC Adv., 2024,14, 15554-15559
https://doi.org/10.1039/D4RA02213K
Catalytic asymmetric carbenoid α-C–H insertion of ether
Significant advancements have been made in catalytic asymmetric α-C–H bond functionalization of ethers via carbenoid insertion over the past decade.

RSC Adv., 2024,14, 15167-15177
https://doi.org/10.1039/D4RA02206H
KI mediated one-pot cascade reaction for synthesis of 1,3,4-selenadiazoles
A one-pot pseudo three-component cascade reaction for the preparation of 1,3,4-selenadiazole from aldehydes, hydrazine and elemental selenium is demonstrated here using the catalytic system consisting of KI and K2S2O8 in DMSO.

RSC Adv., 2024,14, 15449-15454
https://doi.org/10.1039/D4RA01994F
Correction: DCID-mediated esterification of carboxylic acids with alcohols under mild conditions
RSC Adv., 2024,14, 15200-15200
https://doi.org/10.1039/D4RA90051K
Developments and applications of α-bromonitrostyrenes in organic syntheses
In this work, we have described the use of α-bromonitrostyrenes in the synthesis of a wide variety of carbocyclic and heterocyclic compounds under organocatalysis, metal catalysis, and base-catalysis systems as well as catalyst-free reactions.

RSC Adv., 2024,14, 14835-14846
https://doi.org/10.1039/D4RA02474E
Advancements in double decarboxylative coupling reactions of carboxylic acids
The double decarboxylative coupling reaction between two (similar or different) molecules of carboxylic acids is an emerging area that has gained considerable attention as a new avenue for forging carbon–carbon bonds.

RSC Adv., 2024,14, 14919-14933
https://doi.org/10.1039/D4RA01747A
Recent progress and prospects in the organocatalytic Morita–Baylis–Hillman reaction
The organocatalytic Morita–Baylis–Hillman (MBH) reaction, one of the most significant carbon–carbon bond formations involves the addition of α,β-unsaturated carbonyl compounds to activated alkenes to give α-methylene-β-hydroxycarbonyl compounds.

RSC Adv., 2024,14, 14949-14963
https://doi.org/10.1039/D4RA02369B
Three-component synthesis of pyran-fused biscoumarins: an entry to pyridinone- and pyranone-fused coumarins
A base-mediated, three-component synthesis of symmetric and unsymmetric pyran-fused biscoumarins via the coupling of 4-hydroxycoumarin with 4-chloro-3-formylcoumarin in ethanol is reported.

RSC Adv., 2024,14, 14767-14774
https://doi.org/10.1039/D4RA01681E
Photoredox-catalyzed sulfonylation of diaryliodonium salts with DABSO and silyl enolates involving the insertion of SO2
A versatile photoredox-catalyzed three-component sulfonylation of diaryliodonium salts with DABSO and silyl enolates involving the insertion of SO2 for the synthesis of β-keto sulfones was developed.

RSC Adv., 2024,14, 14697-14701
https://doi.org/10.1039/D4RA02773F
Development of novel transition metal-catalyzed synthetic approaches for the synthesis of a dihydrobenzofuran nucleus: a review
The dihydrobenzofuran scaffolds demonstrate a wide range of biological activities. Several transition metals have been employed as catalysts for the efficacious synthesis of these structurally important frameworks.

RSC Adv., 2024,14, 14539-14581
https://doi.org/10.1039/D4RA01830C
Ts2O mediated deoxygenative C2-dithiocarbamation of quinoline N-oxides with CS2 and amines
A Ts2O promoted deoxygenative C2-dithiocarbamation of quinoline N-oxides with CS2 and amines toward various quinoline-dithiocarbamates under transition-metal free conditions was developed.

RSC Adv., 2024,14, 14465-14469
https://doi.org/10.1039/D4RA02003K
Visible-light-induced N-alkylation of anilines with 4-hydroxybutan-2-one
The synthesis of amines through N-alkylation is particularly attractive.

RSC Adv., 2024,14, 14452-14455
https://doi.org/10.1039/D4RA01339E
An easy and simple method for the immobilization of dyes through click reactions: activated alkyne, copper not needed
The copper-free azide–alkyne click reaction has shown to be a successful alternative to immobilize covalently a fluorescente compound onto poly(-L-lactic) acid (PLLA) surfaces.

RSC Adv., 2024,14, 14289-14295
https://doi.org/10.1039/D4RA01776E
Novel isatin–triazole based thiosemicarbazones as potential anticancer agents: synthesis, DFT and molecular docking studies
Synthesis of mono- and bis-thiosemicarbazones 4a–h and 5a–h of isatin–triazole hybrids 3a and 3b in turn accessed via CuAAC, their DFT studies and potential as phosphoinositide 3-kinase (PI3K) inhibitors has been evaluated in this study.

RSC Adv., 2024,14, 14051-14067
https://doi.org/10.1039/D4RA01937G
Lewis acid-mediated transformations of 5-acyl-N-fluoroalkyl-1,2,3-triazoles to cyclopentenones, indenones, or oxazoles
2-N-Substituted indenones, cyclopentenones or 4-carbonyl oxazoles are prepared from 5-acylated N-fluoroalkyl substituted 1,2,3-triazoles and Lewis acids.

RSC Adv., 2024,14, 13640-13645
https://doi.org/10.1039/D4RA01707B
Reactivity of phenoxathiin-based thiacalixarenes towards C-nucleophiles
Phenoxathiin-based macrocycles react with RLi reagents to form a number of different products resulting from cleavage of the calixarene backbone or heterocyclic group.

RSC Adv., 2024,14, 13463-13473
https://doi.org/10.1039/D4RA02524E
New multiple-layered 3D polymers showing aggregation-induced emission and polarization
An exceptional achiral and chiral multilayer 3D polymer has been created and controlled by uniform and distinct aromatic chromophore units that are multiply sandwiched by naphthyl berths.

RSC Adv., 2024,14, 13342-13350
https://doi.org/10.1039/D4RA02128B
Synthesis of novel phthalazine-based derivatives with potent cytotoxicity against HCT-116 cells through apoptosis and VEGFR2 inhibition
A novel phthalazine derivative exhibited potent cytotoxicity against HCT-116 cells as VEGFR2 inhibitor and apoptosis cell death.

RSC Adv., 2024,14, 13027-13043
https://doi.org/10.1039/D4RA02103G
Mono and di ortho-C–H acetoxylation of 2-aryloxyquinoline-3-carbaldehydes
A novel protocol for introducing an acetoxy functional group selectively on the ortho aryl sp2 carbon of 2-aryloxyquinoline-3-carbaldehydes in good yields with good functional group tolerance using a palladium catalyst was developed for the first time.
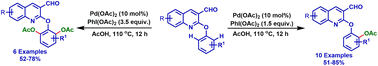
RSC Adv., 2024,14, 13306-13310
https://doi.org/10.1039/D4RA01289E
Synthesis molecular docking and DFT studies on novel indazole derivatives
3-Carboxamide indazoles have been developed using the amide coupling process. Density function theory (DFT) computations, and the assessment of binding energy using Auto Dock, illustrate the pharmaceutical effectiveness.
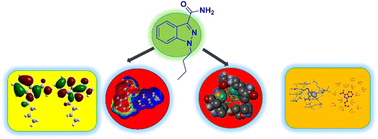
RSC Adv., 2024,14, 13218-13226
https://doi.org/10.1039/D4RA02151G
Grind, shine and detect: mechanochemical synthesis of AIE-active polyaromatic amide and its application as molecular receptor of monovalent anions or nucleotides
The mechanochemical synthesis of AIE-active amide, as well as the application of the title compound as molecular receptor of monovalent anions and nucleotides, are described.

RSC Adv., 2024,14, 13227-13236
https://doi.org/10.1039/D4RA02129K
Browse by Subject
- All (342 articles)
- Atomic/elemental (14 articles)
- Bioanalytical (66 articles)
- Chemometrics (30 articles)
- Crystallography (165 articles)
- Electroanalytical (54 articles)
- Imaging/microscopy (33 articles)
- Mass spectrometry (22 articles)
- Medical diagnostics (80 articles)
- Microfluidics (18 articles)
- Nanoanalysis (21 articles)
- Separation science (28 articles)
- Spectroscopy (97 articles)
- All (283 articles)
- Bioinorganic chemistry (51 articles)
- Bioorganic chemistry (27 articles)
- Biotechnology (54 articles)
- Cellular chemistry (57 articles)
- Computational (83 articles)
- Drug delivery (81 articles)
- Drug discovery (187 articles)
- Imaging/diagnostics (16 articles)
- Molecular biology (6 articles)
- Nanotechnology (45 articles)
- Natural products (29 articles)
- Pharmacology (89 articles)
- Photobiology (15 articles)
- Polymorphism (pharma) (1 article)
- Structural biology (15 articles)
- Toxicology (5 articles)
- All (567 articles)
- Biomaterials (106 articles)
- Biopolymers (103 articles)
- Carbon materials (102 articles)
- Composites (254 articles)
- Electronic materials (97 articles)
- Encapsulation (1 article)
- Energy applications (87 articles)
- Films/membranes (82 articles)
- Gels & soft matter (35 articles)
- Inorganic materials (50 articles)
- Medical materials (72 articles)
- Nanomaterials (121 articles)
- Optical materials (54 articles)
- Organic materials (62 articles)
- Polymers (140 articles)
- All (356 articles)
- Assembly (13 articles)
- Biotechnology (32 articles)
- Carbon nanomaterials (80 articles)
- Imaging/microscopy (18 articles)
- Nanoanalysis (42 articles)
- Nanocatalysis (32 articles)
- Nanomaterials (217 articles)
- Nanomedicine (46 articles)
- Nanotoxicology (58 articles)
- Optical nanomaterials (38 articles)
- Synthesis (13 articles)
- All (177 articles)
- Bioorganic (17 articles)
- Catalysis (48 articles)
- Fine chemicals (25 articles)
- Natural products (21 articles)
- Physical organic (7 articles)
- Stereochemistry (12 articles)
- Supramolecular (3 articles)
- Sustainable synthesis (35 articles)
- Synthetic methodology (106 articles)
- Total synthesis (12 articles)
- All (321 articles)
- Biophysics (3 articles)
- Charge transfer (31 articles)
- Electrochemistry (12 articles)
- Energy research (18 articles)
- Imaging/microscopy (5 articles)
- Kinetics & dynamics (40 articles)
- Materials (108 articles)
- Mechanics (35 articles)
- Nanoscience (72 articles)
- Photoscience (25 articles)
- Quantum & theoretical (88 articles)
- Simulations (25 articles)
- Single molecules (43 articles)
- Soft matter (5 articles)
- Spectroscopy (7 articles)
- Surfaces & interfaces (69 articles)