Mono and di ortho-C–H acetoxylation of 2-aryloxyquinoline-3-carbaldehydes†
Abstract
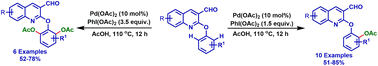
2-Aryloxyquinolines are well known for various biological activities. In this report, we have developed a novel protocol for introducing an acetoxy functional group on the aryl sp2 carbon of 2-aryloxyquinoline-3-carbaldehyde using a palladium catalyst for the first time. Interestingly, this C–H acetoxylation reaction is highly chemo- and site-selective. By modifying the reaction conditions, mono or di ortho-C–H acetoxylation products have been synthesized selectively with good yields and with good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...