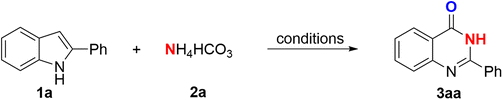Open Access Article
Open Access ArticleIodine-promoted amide formation via oxidative cleavage of indoles: novel quinazoline-4(3H)-one and tryptanthrin syntheses†
Phong Q. Le *af,
Van M. Dinh
*af,
Van M. Dinh b,
Huong D. T. Nguyen
b,
Huong D. T. Nguyen cf,
Hiep Q. Ha
cf,
Hiep Q. Ha *d and
Thanh V. Truong*ef
*d and
Thanh V. Truong*ef
aSchool of Biotechnology, International University-VNU HCM, Quarter 6, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Vietnam. E-mail: lqphong@hcmiu.edu.vn
bDepartment of Chemistry, Chemical-Biological Centre, Umeå University, SE-90187 Umeå, Sweden
cFaculty of Chemistry, University of Science, 227 Nguyen Van Cu, District 5, Ho Chi Minh City, Vietnam
dInstitute of Applied Technology and Sustainable Development, Nguyen Tat Thanh University, Ho Chi Minh City 755414, Vietnam. E-mail: hiepquangha@gmail.com
eFaculty of Chemical Engineering, Ho Chi Minh City University of Technology, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: truongvuthanh@gmail.com
fVietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Vietnam
First published on 14th May 2024
Abstract
A highly efficient method for the direct construction of amide bonds via a selective cleavage of C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in indole structures using an iodine-promoted approach was developed. Mechanistic studies indicated the formation of superoxide radicals obtained from molecular oxygen activation as a key intermediate step, which provided a precursor for subsequent oxidative ring-opening and intermolecular cyclization. A broad range of quinazolin-4(3H)-ones and tryptanthrins were synthesized in moderate to good yields under mild and environmentally benign conditions.
C bonds in indole structures using an iodine-promoted approach was developed. Mechanistic studies indicated the formation of superoxide radicals obtained from molecular oxygen activation as a key intermediate step, which provided a precursor for subsequent oxidative ring-opening and intermolecular cyclization. A broad range of quinazolin-4(3H)-ones and tryptanthrins were synthesized in moderate to good yields under mild and environmentally benign conditions.
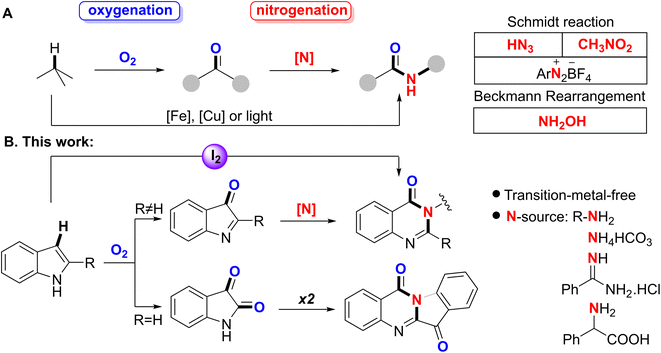
1. Introduction
Amide bonding is important to all forms of life and has been discovered in various natural processes of both abiogenesis and biogenesis.1 Besides biosynthesis, a few synthetic efforts could achieve biomimetic amide production by activation and/or cleavage of C–H bonds.2 A common strategy is to employ an oxidation process in the presence of molecular oxygen,3 followed by the procurement of a nitrogen atom and cleavage of C–C bonds according to the Schmidt reaction4 or Beckmann rearrangement5 to enable the amide bond to be produced. Moreover, Fe6 or Cu-based7 systems and light-assisted8 methods also permit the direct synthesis of amides using hydrocarbon sources (Scheme 1A). However, the research field is still limited, which might be due to the recalcitrance of C–H bonds to being cleaved and the activation of dioxygen in the gas phase under transition metal-free conditions. Herein, we selected the labile C–Hβ of naturally occurring indole structures to investigate the potential in amide bonding. The synthesis employed molecular oxygen and ammonium salts as O- and N-donors, respectively, together with a transition metal-free catalytic system of iodine. We aimed to develop a novel ‘green’ protocol to cleave a C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3–H system for the formation of the C2–N–C3
C3–H system for the formation of the C2–N–C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O combination to obtain quinazolin-4(3H)-ones9 and tryptanthrins10 (Scheme 1B).
O combination to obtain quinazolin-4(3H)-ones9 and tryptanthrins10 (Scheme 1B).
2. Results and discussion
At the beginning, the reaction was found to easily access ammonium to target the desired products. Hence, 2-phenylindole and ammonium hydrogen carbonate were chosen as model substrates to explore the reaction conditions (Table 1). Several additives were investigated for the catalytic role (entries 1–6). To our delight, iodine was found to be the most efficient catalyst for this transformation with a reaction yield of up to 65% (entry 6). The use of N-iodosuccinimide could afford a low yield of the product (36%), while the presence of oxone, I2O5, or iodide salts did not support the reaction. When it came to the solvent selection, ethyl acetate (entry 8) overweighed other common solvents (entries 6 and 7) in enhancing the transformation with 94% yield and possessing several benefits as a cheap, readily available, and less hazardous solvent. Noticeably, no reaction occurred without iodine (entry 9) or K2CO3 (entry 10), indicating the importance of these molecules in the occurrence of the reaction. Replacing K2CO3 with KOH (entry 11) or t-BuOK (entry 12) did not improve the yield, suggesting that the transformation yield was not decided by a very high alkalinity. The amount of iodine used in the reaction had a strong effect on the reaction efficiency, which indicated that using a low amount of catalyst, 0.6 equiv., resulted in no desired product (entry 13). Finally, the experiment revealed that the air atmosphere led to a decreased yield of 3aa (entry 14), suggesting that O2 played an important role in the outcome of the reaction. Thus, the optimal conditions were obtained as follows: indole and NH4HCO3 (1.5 equiv.) with 1.2 equiv. of I2, and 2 equiv. of K2CO3 as the base in ethyl acetate (2 mL) under an O2 atmosphere at 80 °C for 4 h.| Entry | Additive | Base | Solvent | Yield (%) |
|---|---|---|---|---|
| a Standard conditions: 1a (0.15 mmol), 2a (1.5 equiv.), I2 (1.2 equiv.), K2CO3 (2 equiv.), O2, 80 °C, 4 h, solvent (2 mL). All yields were isolated yields.b Being run at 100 °C for 24 h.c Used 0.6 equiv. of I2.d Under air. | ||||
| 1b | Oxone | K2CO3 | CH3NO2 | — |
| 2 | KI | K2CO3 | DMSO | — |
| 3 | NH4I | K2CO3 | DMSO | — |
| 4 | NIS | K2CO3 | DMSO | 36 |
| 5 | I2O5 | K2CO3 | DMSO | — |
| 6 | I2 | K2CO3 | DMSO | 65 |
| 7 | I2 | K2CO3 | Toluene | 42 |
| 8 | I2 | K2CO3 | Ethyl acetate | 94 |
| 9 | — | K2CO3 | Ethyl acetate | — |
| 10 | I2 | — | Ethyl acetate | — |
| 11 | I2 | KOH | Ethyl acetate | — |
| 12 | I2 | t-BuOK | Ethyl acetate | 39 |
| 13c | I2 | K2CO3 | Ethyl acetate | — |
| 14d | I2 | K2CO3 | Ethyl acetate | 63 |
Interestingly, indole derivatives without C2-substitution allowed for a dimerization of the substrate to produce another product, tryptanthrin. The transformation proceeded in the absence of an external nitrogen donor, with the optimal condition of TBHPaq. (3 equiv.), I2 (50 mol%), K2CO3 (2 equiv.), and 3 mL of solvent (ESI, Table S1†).
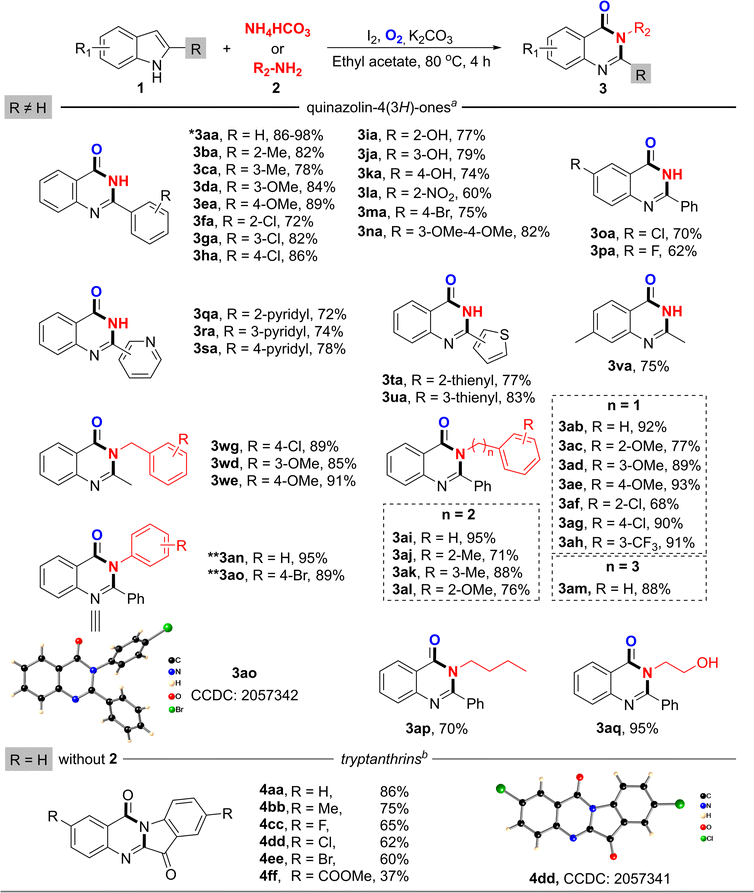
With the optimized reaction conditions available, the scope for this iodine-promoted protocol was then investigated. Various reactants with different substitution patterns were found to compatibly provide the corresponding products with moderate to excellent yields, and are listed in Scheme 2. Out of the N-donating reagents, it was discovered that benzamidine could be perfectly tolerated to form quinazolin-4(3H)-one 3aa with the maximum isolated yield of 98%. In this case, benzamidine or phenylglycine took part as the same N-source as the ammonium salts. However, NH4HCO3 was chosen for the synthesis of 3ba–3va due to its low cost and availability. It was noteworthy that benzyl amine and a number of its derivatives with different functional groups could be utilized for the preparation of quinazolinones (as 3ab, 3ae, 3ag and 3ah) with good yields of 90–93%. We also screened the scope of the alkyl amines, and found that this protocol could be compatibly applied. Butyl amine and ethanol amine were easily introduced into 2-phenylindole to form 3ap and 3aq with yields of 70% and 95%, respectively. Not only could 2-phenylindole be used as a substrate, but 2-methylindole or other 2-substituted indoles could also play the same role by giving products 3qa–3va and 3we–3wg with good efficiency.
By this simple addition of an oxidant, we easily reached the optimum conditions for the tremendous synthetic stage with anilines as an N-donor. Herein, we continued to explore more substrates for the reaction. It was notable that a broad range of aniline derivatives exhibited potential reactivity for the synthesis of the target products. In this stage of the process, we successfully accomplished the introduction of aniline and 4-bromoaniline, affording 3an and 3ao in 95% and 89% yields, respectively. Notably, the structure of 3ao was confirmed by single-crystal X-ray diffraction analysis.
Furthermore, the substrate scope of the dimerization of indole was investigated. Under the optimized conditions in the absence of a N-donor (2), the condensation proceeded well to form the desired tryptanthrin derivatives. The isolation processes were also performed to determine the isolated yields of the products. As shown in Scheme 2, tryptanthrin 4aa could be obtained in 86% yield under the optimized conditions. When an indole-bearing electron-donating group such as 5-methyl-indole was examined for the reaction, the yield of the desired product 4bb was decreased to 75%. With electron-withdrawing groups (including fluoro, chloro, bromo) at the C-5 position of indole, it was observed that the reaction conditions were very compatible with halogenic indole, which yielded the corresponding products 4cc, 4dd and 4ee in satisfactory yields of 65%, 62% and 60%, respectively. On the other hand, methyl 5-carboxylate indole could also afford the desired 4ff with 37%, but there was a huge drop in yield, which could be due to an electron density decrease in the indole ring. The molecular structure of 4dd was also confirmed by X-ray crystallography.
3. Mechanistic study
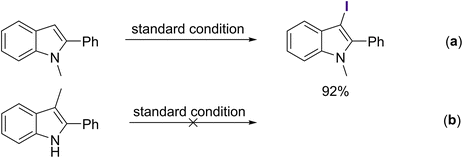
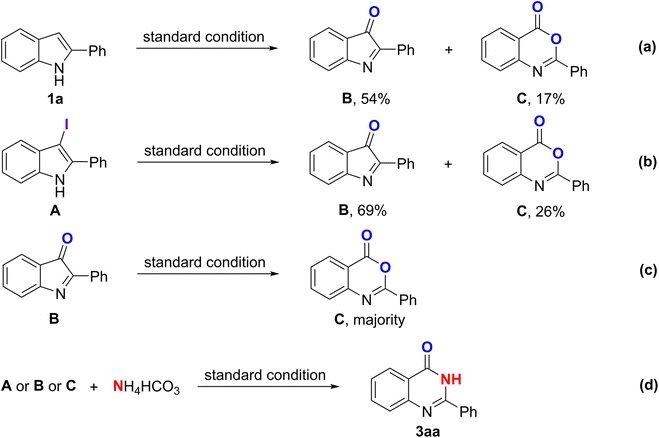
Several control experiments were attempted to better understand the mechanism of this reaction. Initially, a few experiments were conducted to identify which position on the 2-substituted indoles might allow for the attachment of the iodine atom. As observed, N-methyl-2-phenylindole could be directly halogenated to afford its corresponding iodo-substituted derivative on the C-3 position in 92% yield without the use of any N-sources (Scheme 3a). No desired product was recorded when employing the C-3 blocked-2-phenylindole as the starting material (Scheme 3b), showing the importance of the C-3 availability. In addition, 3-iodo-2-phenyl-1H-indole A was detected by GC-MS analysis after running the reaction for 1 h. Typically, the C-3 position of indole exhibits greater nucleophilicity compared to the C-2 position. This phenomenon arises from the electron-donating influence of the nitrogen atom through resonance. Additionally, due to the steric hindrance caused by the C-2 substituent, iodination at the C-3 position becomes a more convenient and favorable reaction pathway. The iodination of 2-phenylindole at the C-3 position may be the key step for this methodology.Under the standard condition in the absence of a nitrogen source, two intermediate compounds, B and C, were recorded with 54% and 17% in yields, respectively (Scheme 4a). Using A as the starting reactant was also substantiated by the formation of those two intermediates (Scheme 4b). Furthermore, the product of the Baeyer–Villiger oxidation expansion was affirmed when B reacted with oxygen under standard conditions (Scheme 4c). All of the intermediates could afford 3aa under standard reaction conditions (Scheme 4d).
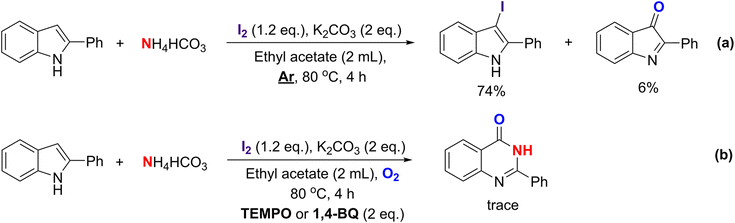
To rationalize the primary role of oxygen, a supplementary experiment has been investigated by carrying out the reaction of 2-phenylindole with ammonium bicarbonate as a nitrogen source under an inert atmosphere (Scheme 5a). Notably, two intermediates including A and B were obtained in 74% and 6% yields, respectively. This reaction nearly stopped at the halogenation step with no further products being recorded, thus partly emphasizing the necessity of oxygen in this transformation. A trace amount of B might come from the presence of oxygen remaining in the solvent. For the radical trapping test, 1a was reacted with NH4HCO3 by using 1.2 equiv. of I2 and 2 equiv. of K2CO3 in the presence of 2 equiv. of radical scavengers, including TEMPO or 1,4-benzoquinone. As a result, only a trace amount of 2-phenylquinazolinone-4(3H)-one was obtained in both cases, suggesting that this reaction would go through the radical pathway (Scheme 5b).
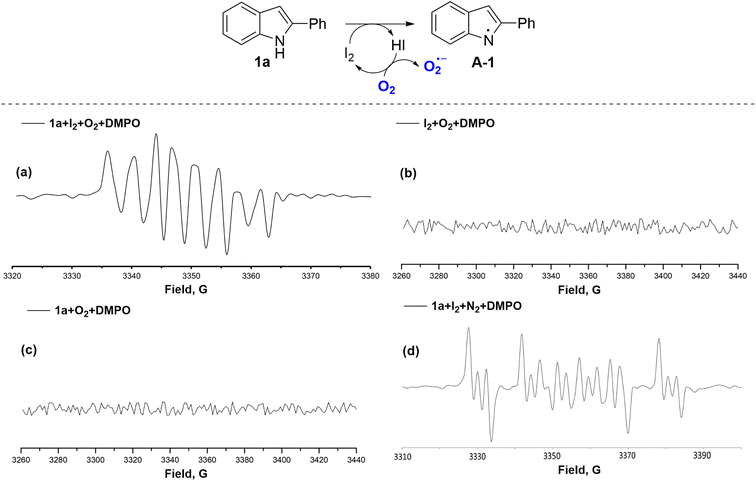
To clarify the radical intermediates of this reaction, electron paramagnetic resonance (EPR) experiments were performed with the addition of the free-radical spin trapping agent, 5,5-dimethyl-1-pyrroline N-oxide (DMPO; Fig. 1 and S1–S7, see ESI† for details). The same signals of superoxide radicals were detected in the reaction mixture of 1a and I2 under the standard condition (Fig. 1a, S1 and S2†), and these signals disappeared in the absence of either (Fig. 1a, b and S3–S6†). Moreover, a different signal appeared in the inert gas atmosphere (N2 or Ar), corresponding to a N-radical (Fig. 1d or S7†). These results showed that 2 did not participate in a radical generation. The N-radical was formed from 1a and then combined with oxygen molecules, creating a superoxide radical.
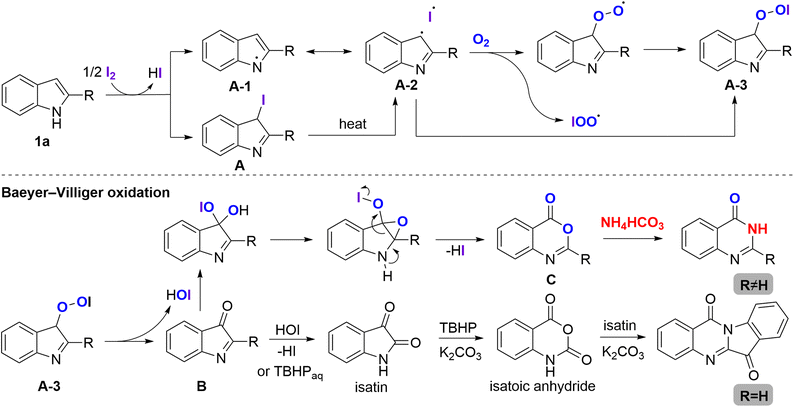
Based on those described experimental results, a plausible mechanism was proposed for the oxidative C2–C3 bond cleavage/amination of indole derivatives (Scheme 6). Initially, iodine would seize an electron from the nitrogen atom of 2-phenylindole to form intermediate A-1, followed by a transformation to A-2. Formation of the iodinated intermediate A from 1a can also be converted to A-2 under heating. Subsequently, this compound was oxidized to afford A-3 and the intermediate 3H-indol-3-one B, followed by the Baeyer–Villiger oxidative expansion to produce benzoxazinone C. Finally, the basicity of the environment somewhat furnished the amination and cyclization, leading to the formation of the final product quinazolinone 3 by the condensation reaction in the presence of a nitrogen source.
In terms of tryptanthrin formation, the oxidation of B by TBHP proceeded to form isatin.11 The nucleophilic attack of TBHP and intramolecular cyclization, followed by a rearrangement, would afford the intermediate isatoic anhydride. Subsequently, the decarboxylative nucleophilic attack of isatoic anhydride with isatin would produce tryptanthrin through an oxidative cyclization.12 Different control experiments on the synthesis of tryptanthrins are described in ESI, Scheme S1.†
4. Conclusion
In conclusion, a completely new synthetic strategy using an I2-promoted domino reaction to achieve sequential C3–H functionalization of indole afforded the formation of an amide bond to access quinazolinones and tryptanthrins. A mechanism with high reliability from EPR and control experiments shows the transformation through an initial radical-mediated pathway, with subsequent Baeyer–Villiger-type oxidation and nitrogen capture under transition-metal-free condition. This developed protocol used a cheap and green catalyst, and diverse available sources of nitrogen under environmentally friendly conditions.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Vietnam National Foundation for Science and Technology Development (NAFOSTED) is acknowledged for financial support via grant no. 104.01-2018.326.References
- (a) R. S. Oremland and C. W. Culbertson, Nature, 1992, 356, 421–423 CrossRef CAS; (b) A. J. Holmes, A. Costello, M. E. Lidstrom and J. C. Murrell, FEMS Microbiol. Lett., 1995, 132, 203–208 CrossRef CAS PubMed; (c) A. J. Holmes, P. Roslev, I. R. McDonald, N. Iversen, K. Henriksen and J. C. Murrell, Appl. Environ. Microbiol., 1999, 65, 3312–3318 CrossRef CAS PubMed; (d) Q. Ma, X. Zhang and Y. Qu, Front. Microbiol., 2018, 9, 2625 CrossRef PubMed.
- (a) S. L. Miller, Science, 1953, 117, 528–529 CrossRef CAS PubMed; (b) S. L. Miller and H. C. Urey, Science, 1959, 130, 245–251 CrossRef CAS PubMed.
- (a) T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329–2364 CrossRef CAS PubMed; (b) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381–3430 RSC; (c) Y.-F. Liang and N. Jiao, Acc. Chem. Res., 2017, 50, 1640–1653 CrossRef CAS PubMed; (d) C. Tang, X. Qiu, Z. Cheng and N. Jiao, Chem. Soc. Rev., 2021, 50, 8067–8101 RSC.
- (a) K. F. Schmidt, Angew. Chem., 1923, 36, 511 Search PubMed; (b) J. Liu, C. Zhang, Z. Zhang, X. Wen, X. Dou, J. Wei, X. Qiu, S. Song and N. Jiao, Science, 2020, 367, 281–285 CrossRef CAS PubMed; (c) F.-T. Meng, Y.-N. Wang, X.-Y. Qin, S.-J. Li, J. Li, W.-J. Hao, S.-J. Tu, Y. Lan and B. Jiang, Nat. Commun., 2022, 13, 7393 CrossRef PubMed.
- K. Kaur and S. Srivastava, New J. Chem., 2020, 44, 18530–18572 RSC.
- C. Qin, W. Zhou, F. Chen, Y. Ou and N. Jiao, Angew. Chem., Int. Ed., 2011, 50, 12595–12599 CrossRef CAS PubMed.
- (a) Y. Feng, Y. Li, G. Cheng, L. Wang and X. Cui, J. Org. Chem., 2015, 80, 7099–7107 CrossRef CAS PubMed; (b) W.-B. Cao, X.-Q. Chu, Y. Zhou, L. Yin, X.-P. Xu and S.-J. Ji, Chem. Commun., 2017, 53, 6601–6604 RSC; (c) W.-B. Cao, B.-B. Liu, X.-P. Xu and S.-J. Ji, Org. Chem. Front., 2018, 5, 1194–1201 RSC.
- L.-L. Zhang, W.-B. Cao, X.-P. Xu and S.-J. Ji, Org. Chem. Front., 2019, 6, 1787–1795 RSC.
- (a) Y. Feng, Y. Li, G. Cheng, L. Wang and X. Cui, J. Org. Chem., 2015, 80, 7099–7107 CrossRef CAS PubMed; (b) S. Mohammed, R. A. Vishwakarma and S. B. Bharate, J. Org. Chem., 2015, 80, 6915–6921 CrossRef CAS PubMed; (c) F.-C. Jia, Z.-W. Zhou, C. Xu, Y.-D. Wu and A.-X. Wu, Org. Lett., 2016, 18, 2942–2945 CrossRef CAS PubMed; (d) L.-C. Wang, S. Du, Z. Chen and X.-F. Wu, Org. Lett., 2020, 22, 5567–5571 CrossRef CAS PubMed; (e) J. He, J. Dong, L. Su, S. Wu, L. Liu, S.-F. Yin and Y. Zhou, Org. Lett., 2020, 22, 2522–2526 CrossRef CAS PubMed; (f) J. Luo, J. Wan, L. Wu, L. Yang and T. Wang, J. Org. Chem., 2022, 87, 9864–9874 CrossRef CAS PubMed.
- (a) J. Hong, M. Zhang, L. Shi, P. Liu, Y. Guo, T. Zhao, Q. Li and L. Yang, New J. Chem., 2022, 46, 13540–13545 RSC; (b) C. Zhang, S. Li, F. Bureš, R. Lee, X. Ye and Z. Jiang, ACS Catal., 2016, 6, 6853–6860 CrossRef CAS; (c) Z. Guo, Y. Liu, H. Li, J. Wu, M. Xie and J. Zhang, Adv. Synth. Catal., 2024, 366, 225 CrossRef CAS; (d) M. Pattarawarapan, N. Wiriya, S. Hongsibsong and W. Phakhodee, J. Org. Chem., 2020, 85, 15743–15751 CrossRef CAS PubMed.
- Y. Zi, Z.-J. Cai, S.-Y. Wang and S.-J. Ji, Org. Lett., 2014, 16, 3094–3097 CrossRef CAS PubMed.
- F.-C. Jia, Z.-W. Zhou, C. Xu, Y.-D. Wu and A.-X. Wu, Org. Lett., 2016, 18, 2942–2945 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2057341 and 2057342. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra01807a |
| This journal is © The Royal Society of Chemistry 2024 |