Themed collection Harnessing non-covalent interactions for synthesis and catalysis

Concluding remarks: Harnessing non-covalent interactions for synthesis and catalysis
An overview of the Faraday Discussion meeting on harnessing non-covalent interactions for synthesis and catalysis, is presented.

Faraday Discuss., 2023,244, 455-458
https://doi.org/10.1039/D3FD90014B
Ultrafast electronic, infrared, and X-ray absorption spectroscopy study of Cu(I) phosphine diimine complexes
The study aims to understand the role of the transient bonding in the interplay between the structural and electronic changes in heteroleptic Cu(I) diimine diphosphine complexes.

Faraday Discuss., 2023,244, 391-410
https://doi.org/10.1039/D3FD00027C
Spiers Memorial Lecture: Shielding the active site: a streptavidin superoxide-dismutase chimera as a host protein for asymmetric transfer hydrogenation
We have evaluated chimeric streptavidin superoxide dismutase C as a scaffold for an asymmetric transfer hydrogenase, incorporating [Cp*Ir(biot-p-L)Cl] as a cofactor.

Faraday Discuss., 2023,244, 9-20
https://doi.org/10.1039/D3FD00034F
Mapping the photochemistry of cyclopentadiene: from theory to ultrafast X-ray scattering
The photochemistry of cyclopentadiene is investigated using nonadiabatic dynamics simulations. Observables for ultrafast X-ray scattering experiments are predicted and mapped onto the structural dynamics.

Faraday Discuss., 2023,244, 269-293
https://doi.org/10.1039/D2FD00176D
A substrate descriptor based approach for the prediction and understanding of the regioselectivity in caged catalyzed hydroformylation
To study whether the use of substrate descriptors to rationalize a catalytic outcome may be an effective tool, we investigated both an encapsulated and non-encapsulated rhodium based catalyst in the hydroformylation reaction of 41 terminal alkenes.

Faraday Discuss., 2023,244, 169-185
https://doi.org/10.1039/D3FD00023K
Structural modifications to platinum(II) pincer complexes resulting in changes in their vapochromic and solvatochromic properties
Platinum pincer complexes display thermochromism or vapochromism and solvatochromism with a range of volatiles and water. Crystal structure analyses show the presence of solvent filled channels and hydrogen bonding interactions that determine the Pt/Pt stacking distance.

Faraday Discuss., 2023,244, 411-433
https://doi.org/10.1039/D3FD00025G
Catalytic templated length-controlled oligomerization
Design of a catalytic, templated length-controlled oligomerization.

Faraday Discuss., 2023,244, 119-133
https://doi.org/10.1039/D3FD00002H
A comparison of non-covalent interactions in the crystal structures of two σ-alkane complexes of Rh exhibiting contrasting stabilities in the solid state
Non-covalent interactions in the Rh σ-alkane complexes [(Cy2PCH2CH2PCy2)Rh(norbornane)][BArF4] and [(Cy2PCH2CH2PCy2)Rh(propane)][BArF4] correlate with their stability in the solid-state.

Faraday Discuss., 2023,244, 222-240
https://doi.org/10.1039/D3FD00009E
Probing the influence of substrate binding on photocatalytic dehalogenation with a heteroleptic supramolecular [M4La2Lb2] square containing PDI photosensitizers as ligands
A heteroleptic supramolecular square containing perylene-diimide moieties features photocatalytic activity for dehalogenation reactions, influenced by substrate binding.
![Graphical abstract: Probing the influence of substrate binding on photocatalytic dehalogenation with a heteroleptic supramolecular [M4La2Lb2] square containing PDI photosensitizers as ligands](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D2FD00179A&imageInfo.ImageIdentifier.Year=2023)
Faraday Discuss., 2023,244, 199-209
https://doi.org/10.1039/D2FD00179A
H-Bonding leading to latent initiators for olefin metathesis polymerization
Ruthenium–NHC based catalysts, with a chelated iminium ligand trans to the NHC ligand that polymerize DCPD at different temperatures are studied using DFT calculations to unveil the reaction mechanism.

Faraday Discuss., 2023,244, 252-268
https://doi.org/10.1039/D2FD00163B
Noncovalent bonding assessment by pair distribution function
X-ray pair distribution function analyses can improve our understanding of local structural deviations resulting from noncovalent bonds and guide the development of novel functional materials.

Faraday Discuss., 2023,244, 356-369
https://doi.org/10.1039/D2FD00159D
Catalytic activation via π-backbonding in halogen bonds
Halogen bonding (XB) could help lower the activation barrier of reactions through nucleophilic modulation, a technique rarely explored previously but demonstrated here by this proof-of-concept study.

Faraday Discuss., 2023,244, 241-251
https://doi.org/10.1039/D2FD00140C
Boosting the activity of Mizoroki–Heck cross-coupling reactions with a supramolecular palladium catalyst favouring remote Zn⋯pyridine interactions
Kinetically labile Zn⋯N interactions between substrate and catalyst are responsible for the enhanced reactivity as well as substrate selectivity disclosed in a supramolecular palladium-catalyzed Mizoroki–Heck reaction between bromopyridines and olefins.
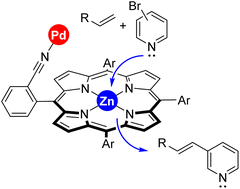
Faraday Discuss., 2023,244, 186-198
https://doi.org/10.1039/D2FD00165A
Chalcogen bonding in copper(II)-mediated synthesis
Chalcogen bonding as a synthetic tool in metal-mediated synthesis and engineering of the secondary coordination sphere of copper(II) complexes.

Faraday Discuss., 2023,244, 77-95
https://doi.org/10.1039/D2FD00160H
Site-selective methylene C–H oxidation of an alkyl diamine enabled by supramolecular recognition using a bioinspired manganese catalyst
A manganese catalyst equipped with 18-benzo-6-crown ether receptors has been employed in the catalytic oxidation of tetradecane-1,14-diamine. Binding of the protonated amines results in selective (up to 92%) oxidation of the C6/C7 methylenic sites.

Faraday Discuss., 2023,244, 51-61
https://doi.org/10.1039/D2FD00177B
Machine-learning based prediction of small molecule–surface interaction potentials
We present a flexible artificial neural network model for the prediction of small molecule–surface interaction potentials including medium effects.

Faraday Discuss., 2023,244, 306-335
https://doi.org/10.1039/D2FD00155A
Uncovering the role of non-covalent interactions in solid-state photoswitches by non-spherical structure refinements with NoSpherA2
Charge density analysis, via Hirshfeld atom refinement in NoSpherA2, is used to rationalise key structure–property relationships in photoswitchable single-crystals.

Faraday Discuss., 2023,244, 370-390
https://doi.org/10.1039/D2FD00158F
Effect of liquid confinement on regioselectivity in the hydrosilylation of alkynes with cationic Rh(I) N-heterocyclic carbene catalysts
A liquid confinement is created using an ionic liquid containing a Rh–N-heterocyclic carbene catalyst, all supported on a polymeric monolithic support. Alkyne hydrosilylation reactions can be run under biphasic conditions with high β-(Z) selectivity.

Faraday Discuss., 2023,244, 39-50
https://doi.org/10.1039/D2FD00152G
Solution and solid-state studies of hydrogen and halogen bonding with N-heterocyclic carbene supported nickel(II) fluoride complexes
Halogen and hydrogen bonding using bis(carbene) nickel fluoride complexes as acceptors and suitable halogen and hydrogen bond donors is presented, showing interactions that are much stronger than those of related phosphine supported nickel fluorides.

Faraday Discuss., 2023,244, 62-76
https://doi.org/10.1039/D2FD00171C
On the mechanism of intermolecular nitrogen-atom transfer from a lattice-isolated diruthenium nitride intermediate
Computational studies indicate nitrogen-atom transfer at lattice-confined diruthenium sites proceeds by an H-atom abstraction, radical rebound mechanism and corroborate available porosity-dependent kinetic isotope effects.

Faraday Discuss., 2023,244, 154-168
https://doi.org/10.1039/D2FD00167E
The bridge towards a more stable and active side-on-peroxido (Cu2II(µ-η2:η2-O2)) complex as a tyrosinase model system
A novel dinucleating bis(pyrazolyl)methane ligand was developed for tyrosinase model systems.

Faraday Discuss., 2023,244, 134-153
https://doi.org/10.1039/D2FD00162D
Alkali metal⋯methyl short contacts in aluminates: more than agostic interactions
Short contacts between alkali metal cations and methyl groups in aluminates, traditionally considered agostic interactions, can be rationalized in terms of an electron-rich carbon atom and its interaction with the cation.

Faraday Discuss., 2023,244, 294-305
https://doi.org/10.1039/D2FD00144F
Fine-tuning non-covalent interactions between hybrid metal-oxo clusters and proteins
Interactions between lysozyme and hybrid Anderson–Evans polyoxometalate clusters reveal the synergistic contributions of the metal-oxo core and organic ligands towards non-covalent protein binding, allowing for specific interactions to be tuned.

Faraday Discuss., 2023,244, 21-38
https://doi.org/10.1039/D2FD00161F
Make – underpinning concepts of the synthesis of systems where non-covalent interactions are important: general discussion
Faraday Discuss., 2023,244, 434-454
https://doi.org/10.1039/D3FD90012F
Model – state-of-the-art modelling and computational analysis of reactive sites: general discussion
Faraday Discuss., 2023,244, 336-355
https://doi.org/10.1039/D3FD90015K
Measure – understanding of structural and electronic changes occurring within the relevant timescale of catalytic systems: general discussion
Faraday Discuss., 2023,244, 210-221
https://doi.org/10.1039/D3FD90016A
Manipulate – techniques to manipulate the surroundings of a synthetic catalyst to control activity and selectivity: general discussion
Faraday Discuss., 2023,244, 96-118
https://doi.org/10.1039/D3FD90013D
About this collection
We are delighted to share with you a selection of the papers associated with a Faraday Discussion on Harnessing non-covalent interactions for synthesis and catalysis. More information about the related event may be found here: http://rsc.li/noncovalent-fd2023. Additional articles will be added to the collection as they are published. The final versions of all the articles presented and a record of the discussions will be published after the event.
This meeting will bring together researchers from across the physical and life sciences working in the areas of synthesis, materials and catalysis and will be of particular interest to those interested in the mechanisms of both solid-state and solution processes where non-covalent interactions are important. There will be a particular emphasis on understanding and controlling the non-covalent intermolecular interactions that drive synthetic and catalytic processes across a range of length and timescales. A common theme will be the development of new synthetic, measurement and modelling techniques that can be applied to new areas, with the overall aim of understanding and designing new efficient processes in chemistry, materials and biology. The meeting will embrace topics such as: synthetic methods that allow for control of the non-covalent environment around active sites, how these are identified and quantified, and the consequences of these interactions on structure and catalytic processes (for example rate, stability, selectivity). This meeting will cover 4 main themes: Make – underpinning concepts of the synthesis of systems where non-covalent interactions are important, Measure – understanding of structural and electronic changes occurring within the relevant timescale of catalytic systems, Model – state-of-the-art modelling and computational analysis of reactive sites and Manipulate – techniques to manipulate the surroundings of a synthetic catalyst to control activity and selectivity.
On behalf of the Scientific Committee, we hope you join us and participate in this exciting event, and that you enjoy these articles and the record of the discussion.