Themed collection Celebrating the RAOBC symposium

Fluorescent probes for hydrogen polysulfides (H2Sn, n > 1): from design rationale to applications
Hydrogen polysulfides (H2Sn, n > 1) are gaining much research interest due to their involvement in signaling and cytoprotection. The present review highlights recent advances in the design of fluorescent probes for the detection of H2Sn along with the fundamental challenges and future prospects in this field.
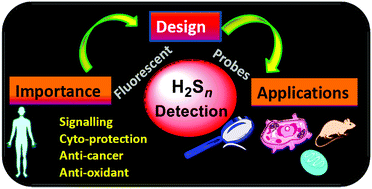
Org. Biomol. Chem., 2017,15, 6692-6701
https://doi.org/10.1039/C7OB01615H
ipso-Cyclization: an emerging tool for multifunctional spirocyclohexadienones
Various ipso-annulation strategies to access diversely substituted spirocyclohexadienones and their applications have been presented.
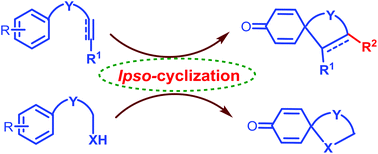
Org. Biomol. Chem., 2017,15, 3130-3151
https://doi.org/10.1039/C7OB00405B
C–H imidation: a distinct perspective of C–N bond formation
The direct imidation strategy proficiently constructs C–N bonds and creates the useful amine functional group in the molecular template.

Org. Biomol. Chem., 2017,15, 1282-1293
https://doi.org/10.1039/C6OB02162J
Aldehydes can switch the chemoselectivity of electrophiles in protein labeling
The derivatization of an electrophile can switch its chemoselectivity. The aldehyde-conjugated epoxide and sulfonate ester provide the proof of principle and deliver N-terminus tagged proteins.

Org. Biomol. Chem., 2018,16, 9377-9381
https://doi.org/10.1039/C8OB02897D
A cascade synthesis of S-allyl benzoylcarbamothioates via Mumm-type rearrangement
A cascade synthesis of S-allyl benzoylcarbamothioates has been achieved from Morita Baylis Hillman alcohols and aroyl isothiocyanates via Mumm-type rearrangement.
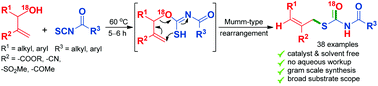
Org. Biomol. Chem., 2018,16, 7787-7791
https://doi.org/10.1039/C8OB02293C
Facile synthesis of triphenylenes and triphenylene/phenanthrene fused heteroaromatics
Facile synthesis of triphenylene fused imidazoles, quinoxaline and phenanthro[9,10-d]-fused pyrazines via CAN-mediated oxidative cyclodehydrogenation.
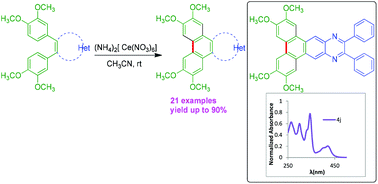
Org. Biomol. Chem., 2018,16, 7134-7138
https://doi.org/10.1039/C8OB01930D
Gold(I)-catalyzed cross-coupling reactions of aryldiazonium salts with organostannanes
Gold(I)-catalyzed cross-coupling reactions of aryldiazonium salts with organostannanes are described.
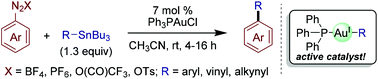
Org. Biomol. Chem., 2018,16, 2865-2869
https://doi.org/10.1039/C8OB00630J
Programmed synthesis of triarylnitroimidazoles via sequential cross-coupling reactions
Transition-metal-catalyzed programmed sequential arylation of 2-chloro-4-nitro-1H-imidazoles was achieved.
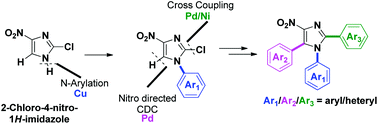
Org. Biomol. Chem., 2019,17, 2134-2147
https://doi.org/10.1039/C9OB00144A
Regiospecific formal [3 + 2] annulation of tert-propargyl alcohols with acyclic 1,3-diketones via the cycloisomerization of homoallenyl ketones
A one-pot, regiospecific synthesis of dihydrofurans bearing a quaternary centre is developed from the formal [3 + 2] annulation of tert-propargyl alcohols and 1,3-diketones through the SN2I mechanism to form homoallenyl ketone and a subsequent cycloisomerization reaction.
![Graphical abstract: Regiospecific formal [3 + 2] annulation of tert-propargyl alcohols with acyclic 1,3-diketones via the cycloisomerization of homoallenyl ketones](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8OB02158A&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 1924-1928
https://doi.org/10.1039/C8OB02158A
Synthesis of quinazoline-3-oxides via a Pd(II) catalyzed azide–isocyanide coupling/cyclocondensation reaction
One-pot three-component synthesis of quinazoline 3-oxides promoted by single Pd(II) catalysis under mild reaction conditions.
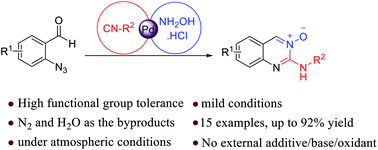
Org. Biomol. Chem., 2019,17, 363-368
https://doi.org/10.1039/C8OB02627K
L-Dopa and dopamine conjugated naphthalenediimides modulate amyloid β toxicity
We report amino acid, L-dopa and dopamine functionalised naphthalenediimides (NDIs) and the detailed in silico and in vitro studies to identify potential multifunctional modulators of amyloid β toxicity.

Org. Biomol. Chem., 2018,16, 7682-7692
https://doi.org/10.1039/C8OB01691G
Enantioselective 1,4-Michael addition reaction of pyrazolin-5-one derivatives with 2-enoylpyridines catalyzed by Cinchona derived squaramides
Cinchonidine derived squaramide catalyzed Michael addition of pyrazolin-5-ones to 2-enoylpyridines providing chiral molecules bearing two heterocyclic motifs with enantiomeric excess up to 96%.
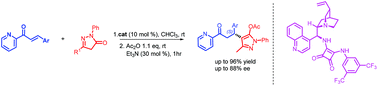
Org. Biomol. Chem., 2018,16, 6470-6478
https://doi.org/10.1039/C8OB01588K
A quantification scheme for non-covalent interactions in the enantio-controlling transition states in asymmetric catalysis
A simple quantification scheme for estimating the strength of non-covalent interactions in the enantio-controlling transition states is proposed.

Org. Biomol. Chem., 2018,16, 5643-5652
https://doi.org/10.1039/C8OB01158C
Highly regioselective, electrophile induced cyclizations of 2-(prop-1-ynyl)benzamides
We report an electrophile promoted, highly regioselective (∼100%) synthesis of 5-membered haloimidiates from 2-(1-alkynyl)benzamides under metal free conditions.

Org. Biomol. Chem., 2018,16, 3947-3951
https://doi.org/10.1039/C8OB00434J
An expeditious route to the synthesis of the enantioenriched tetracyclic core of ergot alkaloids via an organocatalytic aldol reaction
A concise route to the synthesis of the enantiopure tetracyclic scaffold of ergot alkaloids has been developed via a key organocatalytic aldol reaction using paraformaldehyde as the C1-unit in the presence of thiourea ligand followed by a Pd-catalyzed directed coupling.
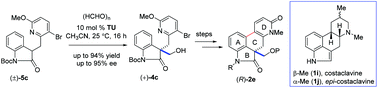
Org. Biomol. Chem., 2018,16, 2427-2437
https://doi.org/10.1039/C7OB03069J
Synthesis of Cu-catalysed quinazolinones using a Csp3–H functionalisation/cyclisation strategy
A series of 2,3-disubstituted-4(3H)-quinazolinones were synthesised via a copper-catalysed Csp3–H functionalisation/cyclisation of 2-amino-N,N-dialkylbenzamides.

Org. Biomol. Chem., 2017,15, 7140-7146
https://doi.org/10.1039/C7OB01723E
“On water” catalytic enantioselective sulfenylation of deconjugated butyrolactams
The first catalytic enantioselective α-sulfenylation of deconjugated butyrolactams has been developed using dimeric cinchona alkaloids as catalysts in water-enriched reaction medium. The applicability of the same catalyst system for enantioselective α-selenylation and formal vinylogous γ-hydroxylation of deconjugated butyrolactam has also been described.
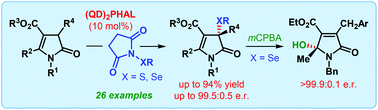
Org. Biomol. Chem., 2017,15, 6921-6925
https://doi.org/10.1039/C7OB01714F
Construction of thiazines and oxathianes via [3 + 3] annulation of N-tosylaziridinedicarboxylates and oxiranes with 1,4-dithiane-2,5-diol: application towards the synthesis of bioactive molecules
Efficient construction of functionalized thiazine and oxathiane derivatives via [3 + 3] annulation of N-tosylaziridinedicarboxylates and oxiranes with in situ generated mercaptoaldehyde in the presence of a Lewis acid is described.
![Graphical abstract: Construction of thiazines and oxathianes via [3 + 3] annulation of N-tosylaziridinedicarboxylates and oxiranes with 1,4-dithiane-2,5-diol: application towards the synthesis of bioactive molecules](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7OB00941K&imageInfo.ImageIdentifier.Year=2017)
Org. Biomol. Chem., 2017,15, 5182-5190
https://doi.org/10.1039/C7OB00941K
Intramolecular Minisci acylation under silver-free neutral conditions for the synthesis of azafluorenones and fluorenones
An intramolecular Minisci acylation under silver-free neutral conditions providing access to azafluorenones and fluorenones has been developed.

Org. Biomol. Chem., 2017,15, 2199-2210
https://doi.org/10.1039/C7OB00077D
About this collection
The Recent Advances in Organic and Bio-organic Chemistry (RAOBC) Symposium, held last month at IISER Mohali, gave a platform to dynamic researchers in organic, bioorganic and medicinal chemistry to share their innovations and inspire fellow researchers. We are delighted to showcase the excellent research being carried out by the RAOBC speakers with this thematic collection, highlighting the fantastic work they have presented in Organic & Biomolecular Chemistry from 2017-2019. We hope you enjoy reading these articles.