An expeditious route to the synthesis of the enantioenriched tetracyclic core of ergot alkaloids via an organocatalytic aldol reaction†‡
Abstract
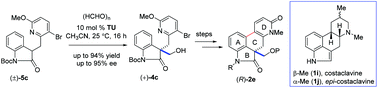
The synthesis of the tetracyclic skeleton of ergot alkaloids has been developed via a key organocatalytic enantioselective aldol reaction using paraformaldehyde as the C1-unit in the presence of thiourea catalyst followed by a key Pd-catalyzed directed coupling accelerated by the DavePhos ligand. Utilizing the aforementioned strategy, we have synthesized a key tetracyclic intermediate in up to 95% ee with high yield.
- This article is part of the themed collection: Celebrating the RAOBC symposium


 Please wait while we load your content...
Please wait while we load your content...