“On water” catalytic enantioselective sulfenylation of deconjugated butyrolactams†
Abstract
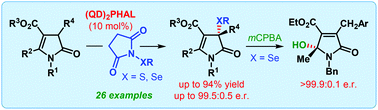
The first catalytic enantioselective α-sulfenylation of deconjugated butyrolactams has been developed using dimeric cinchona alkaloids as catalysts in a water-enriched reaction medium. Highly substituted and densely functionalized γ-lactams, bearing a quaternary stereogenic center, are produced with up to 99.5 : 0.5 er. The applicability of the same catalyst system for the enantioselective α-selenylation and formal vinylogous γ-hydroxylation of deconjugated butyrolactam has also been described.
- This article is part of the themed collection: Celebrating the RAOBC symposium


 Please wait while we load your content...
Please wait while we load your content...