Themed collection Macrocycles with bio-related applications

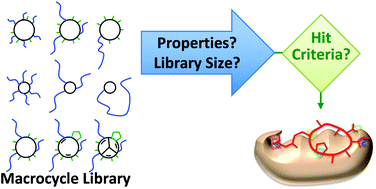
Progress towards the broad use of non-peptide synthetic macrocycles in drug discovery
The broad use of non-peptide synthetic macrocycles in drug discovery is benefitting from recent advances in our understanding of what molecular properties define a useful macrocyclic screening hit.
Org. Biomol. Chem., 2017,15, 7729-7735
https://doi.org/10.1039/C7OB00056A
Generation of a cell-permeable cycloheptapeptidyl inhibitor against the peptidyl–prolyl isomerase Pin1
Integration of Pin1-binding and cell-penetrating sequences results in a cell-permeable, biologically active cycloheptapeptide inhibitor against Pin1.
Org. Biomol. Chem., 2017,15, 4540-4543
https://doi.org/10.1039/C7OB00430C
Comparative pharmacokinetic profile of cyclosporine (CsA) with a decapeptide and a linear analogue
The synthesis and in vivo pharmacokinetic profile of an analogue of cyclosporine is disclosed.
Org. Biomol. Chem., 2017,15, 2501-2506
https://doi.org/10.1039/C7OB00096K
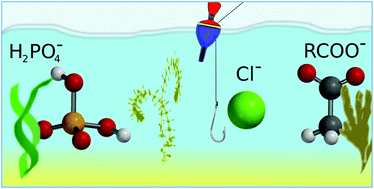
Catching the chloride: searching for non-Hofmeister selectivity behavior in systematically varied polyamide macrocyclic receptors
Searching for regularities in the large set of structurally diverse macrocyclic probes allowed us to determine the structural requirements for the selective recognition of chloride over more basic anions such as H2PO4− or RCO2− by a putative anion receptor.
Org. Biomol. Chem., 2017,15, 5927-5943
https://doi.org/10.1039/C7OB01385J
Macrocyclic peptide inhibitors for the protein–protein interaction of Zaire Ebola virus protein 24 and karyopherin alpha 5
Describes the identification of macrocyclic peptide inhibitors of the ebola VP24 protein–karyopherin alpha 5 protein–protein interaction with nanomolar affinity for VP24.
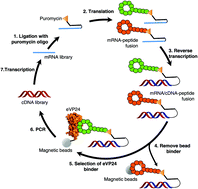
Org. Biomol. Chem., 2017,15, 5155-5160
https://doi.org/10.1039/C7OB00012J
Eight at one stroke – a synthetic tetra-disulfide peptide epitope
A tetra-disulfide peptide dimer, representing an antiparallel hinge, is synthesised without the need for orthogonal cysteine protecting groups.
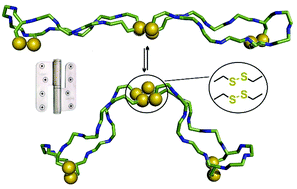
Org. Biomol. Chem., 2017,15, 2512-2521
https://doi.org/10.1039/C6OB02746F
Diversity-oriented synthesis and cytotoxic activity evaluation of biaryl-containing macrocycles
Synthesis of biaryl-containing macrocycles has been carried out through a four-step approach comprising two Ugi four component reactions and a Suzuki–Miyaura macrocyclization.
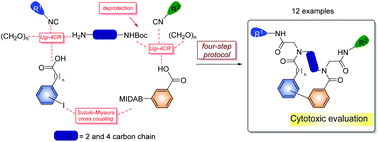
Org. Biomol. Chem., 2017,15, 2450-2458
https://doi.org/10.1039/C6OB02726A
Structure–activity relationship of novel macrocyclic biased apelin receptor agonists
Apelin is the endogenous ligand for the G protein-coupled receptor APJ and exerts a key role in regulating cardiovascular functions.

Org. Biomol. Chem., 2017,15, 449-458
https://doi.org/10.1039/C6OB02247B
Pseudopeptidic compounds for the generation of dynamic combinatorial libraries of chemically diverse macrocycles in aqueous media
The combination of pseudopeptidic dithiol building blocks leads to the generation of highly diverse dynamic libraries of macrocycles in aqueous media.
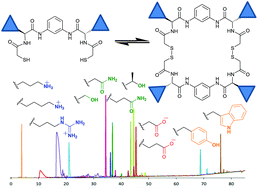
Org. Biomol. Chem., 2017,15, 213-219
https://doi.org/10.1039/C6OB02441F
Downsizing the BAD BH3 peptide to small constrained α-helices with improved ligand efficiency
Using macrocycles to meet the challenge of reducing proteins to smaller molecules that maintain affinity and inhibitory potency for Bcl-xL.
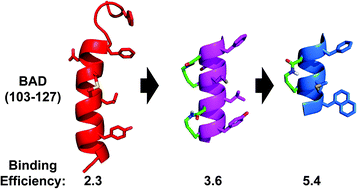
Org. Biomol. Chem., 2016,14, 10939-10945
https://doi.org/10.1039/C6OB02185A
Synthesis and complexing properties of cyclic benzylopeptoids – a new family of extended macrocyclic peptoids
Members of a new class of cyclic “extended” peptoids (the “benzylopeptoids”) efficiently capture sodium ions with different stoichiometries depending on the ring morphology.
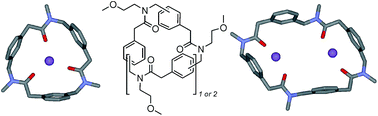
Org. Biomol. Chem., 2016,14, 9055-9062
https://doi.org/10.1039/C6OB01683A
A mechanistic study on the inhibition of α-chymotrypsin by a macrocyclic peptidomimetic aldehyde
NMR and X-ray crystallography reveals covalent attachment of the macrocyclic aldehyde to serine195 of α-chymotrypsin and that its backbone binds as a β-strand.
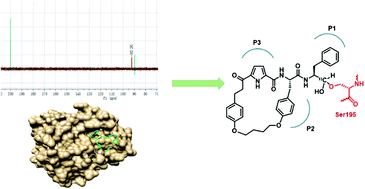
Org. Biomol. Chem., 2016,14, 6970-6978
https://doi.org/10.1039/C6OB01159D
About this collection
This themed collection, Guest Edited by Professor Andrei Yudin, Dr Spiros Liras and Dr Conor Scully is a forum to disseminate the latest findings in the burgeoning field of macrocycles. This will include studies aimed at new ways of constructing macrocycles, efforts to understand their biological activity, and new approaches to deciphering conformational profiles using state of the art spectroscopic methods. This collection is aimed at a broad audience comprising synthetic, medicinal, and biological chemists.
Articles in this themed collection will be added below as soon as possible after they are published.
Please return to this page frequently to see the collection grow.