Eight at one stroke – a synthetic tetra-disulfide peptide epitope†
Abstract
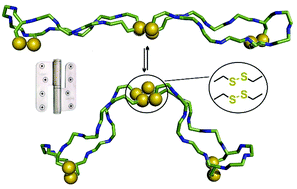
We have designed a cysteine-rich β-hairpin peptide which dimerises spontaneously to the antiparallel double β-hairpin motif C1–C12′, C1′–C12, C5–C8, C5′–C8′-tricyclo-(CHWECCitGCRLVC)2. The highly regioselective oxidation of eight cysteines yields an intermolecular bi-disulfide 24mer hinge peptide from two individual 12mer β-hairpins, each rigidified by an additional intramolecular disulfide bond – all in all a tetra-disulfide. The reaction kinetics of air-oxidation were followed by HPLC and the constitutional isomer was identified by mass spectrometry. The hairpin conformation was characterised in detail by NMR spectroscopy and the opening angle of the antiparallel hinge was estimated from drift times obtained by ion-mobility spectrometry. Based on a set of investigated disulfide motifs, we are able to rationalise how the unbalanced number of bonded and non-bonded hydrogen pairs in a 12 mer hairpin causes their dimerisation. The unique dimeric bi-/tetra-disulfides provide systematic insights into β-hairpin formation. They can serve as a standalone structural element for the oligomerisation of peptide epitopes where structural diversity is generated from a minimal number of amino acids.
- This article is part of the themed collection: Macrocycles with bio-related applications


 Please wait while we load your content...
Please wait while we load your content...