Themed collection The halogen bond: a new avenue in recognition and self-assembly

The halogen bond: a new avenue in recognition and self-assembly
Welcome to this themed issue of NJC entitled: ‘The halogen bond: a new avenue in recognition and self-assembly’.

New J. Chem., 2018,42, 10461-10462
https://doi.org/10.1039/C8NJ90054J
Solvatochromism and fluorescence response of a halogen bonding anion receptor
A pair of 2,6-bis(4-ethynylpyridinyl)-4-fluoroaniline XB and HB receptors display solvatochromic absorption and emission.
New J. Chem., 2018,42, 10489-10492
https://doi.org/10.1039/C8NJ00558C
Cooperative intermolecular S–Cl⋯O and F⋯F associations in the crystal packing of α,ω-di(sulfonyl chloride) perfluoroalkanes, ClSO2(CF2)nSO2Cl, where n = 4, 6
Halogen bonding between neighboring sulfonyl chloride groups and short fluorine–fluorine contacts supports crystal formation in the title compounds.
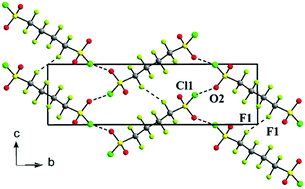
New J. Chem., 2018,42, 10484-10488
https://doi.org/10.1039/C8NJ00536B
A pyrrole-containing cleft-type halogen bonding receptor for oxoanion recognition and sensing in aqueous solvent media
A halogen bonding pyrrole-bis(iodotriazolium) motif facilitates rarely observed augmented binding affinities and selective sensing of oxoanions (H2PO4− and SO42−).
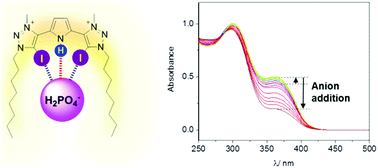
New J. Chem., 2018,42, 10472-10475
https://doi.org/10.1039/C8NJ00420J
Halogen bonding two-point recognition with terphenyl derivatives
Neutral terphenyl-based halogen bond donors form two-point halogen bonding motifs with oxadiazoles in the solid state.
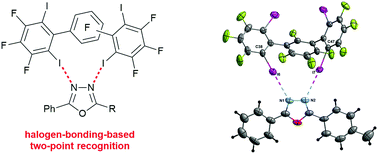
New J. Chem., 2018,42, 10476-10480
https://doi.org/10.1039/C8NJ00962G
Various types of non-covalent interactions contributing towards crystal packing of halogenated diphospha-dicarbaborane with an open pentagonal belt
We have synthesized and crystalized 3-Cl-10-I-nido-7,8,9,11-P2C2B7H7. Quantum chemical calculations have demonstrated that the obtained crystal structure is stabilized by hydrogen, dihydrogen and pnictogen bonds.
New J. Chem., 2018,42, 10481-10483
https://doi.org/10.1039/C8NJ00450A
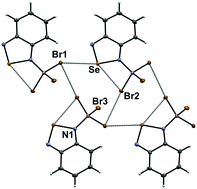
Cyanine dyes: synergistic action of hydrogen, halogen and chalcogen bonds allows discrete I42− anions in crystals
Discrete tetraiodide dianions (I42−) are formed in crystals via halogen bond coordination of I2 by iodide anions which are pinned in their positions by a network of hydrogen bonds involving a benzoselenazole cyanine dye.
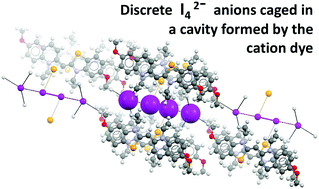
New J. Chem., 2018,42, 10463-10466
https://doi.org/10.1039/C8NJ00421H
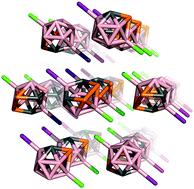
Halogen-bond driven self-assembly of triangular macrocycles
Halogen bonds drive the self-assembly of 2-iodoethynylpyridine and 2- iodoethynyl-1-methyl-imidazole into discrete supramolecular triangles.
New J. Chem., 2018,42, 10467-10471
https://doi.org/10.1039/C8NJ00759D
Ligand-driven formation of halogen bonds involving Au(I) complexes
A theoretical investigation shows that the Au(I) centre in a variety of complexes can behave as a halogen bond acceptor.
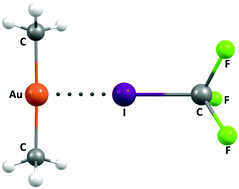
New J. Chem., 2018,42, 10529-10538
https://doi.org/10.1039/C8NJ01510D
Sigma-hole interactions in the molecular and crystal structures of N-boryl benzo-2,1,3-selenadiazoles
Crystallography and DFT reveal competition between chalcogen bonding and other intermolecular interactions.
New J. Chem., 2018,42, 10555-10562
https://doi.org/10.1039/C8NJ00553B
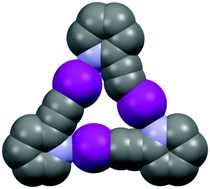
Factors affecting charge transfer in tetraiodide dianions
Thirty-one examples of crystal structures containing discrete tetraiodide I42− dianions were identified from the Cambridge Structural Database (CSD) and analyzed in detail in order to find the factors influencing the geometry of this rare fragment. The intermolecular interactions are at least partially responsible for the changes in the geometry of the dianion.
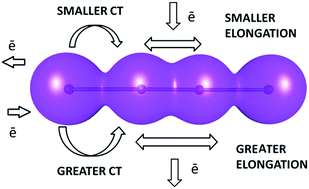
New J. Chem., 2018,42, 10661-10669
https://doi.org/10.1039/C8NJ00737C
S⋯S and S⋯P chalcogen bonding in solution: a cryospectroscopic study of the complexes of 2,2,4,4-tetrafluoro-1,3-dithietane with dimethyl sulfide and trimethylphosphine
Experimental evidence on the formation of S⋯S and P⋯S chalcogen bonded complexes between 2,2,4,4-tetrafluoro-1,3-dithiethane and the Lewis bases dimethyl sulfide and trimethylphosphine is obtained using infrared spectroscopy of solutions in liquid krypton.
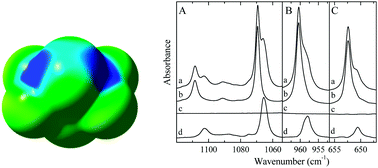
New J. Chem., 2018,42, 10563-10571
https://doi.org/10.1039/C8NJ01648H
Structural and biological evaluation of halogen derivatives of 1,9-pyrazoloanthrones towards the design of a specific potent inhibitor of c-Jun-N-terminal kinase (JNK)
A specifically designed halogen derivatives of anthrapyrazolone for the selective inhibition of JNKs at lower concentrations with minimal off-target effects on MAPKs.
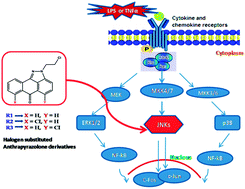
New J. Chem., 2018,42, 10651-10660
https://doi.org/10.1039/C8NJ00852C
Competition between hydrogen bonds and halogen bonds: a structural study
O–H hydrogen-bond donors and R–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–I halogen-bond donors are close competitors for suitable acceptor sites in solid-state assembly.
C–I halogen-bond donors are close competitors for suitable acceptor sites in solid-state assembly.
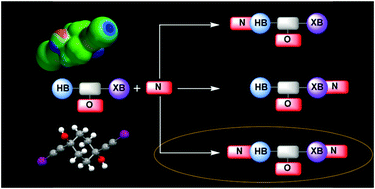
New J. Chem., 2018,42, 10539-10547
https://doi.org/10.1039/C8NJ00537K
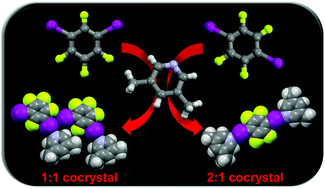
Halogen bonding and triiodide asymmetry in cocrystals of triphenylmethylphosphonium triiodide with organoiodines
Halogen bonding in triiodide–organoiodine cocrystals.
New J. Chem., 2018,42, 10518-10528
https://doi.org/10.1039/C8NJ01373J
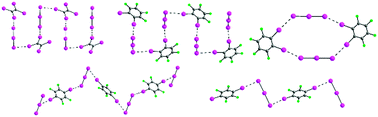
Comparison of isomeric meta- and para-diiodotetrafluorobenzene as halogen bond donors in crystal engineering
The ability of meta- and para-diiodotetrafluorobenzene to act as halogen bond donors in crystal engineering has been compared by the synthesis and crystal structure analysis of a family of 20 novel halogen-bonded cocrystals with simple monotopic and ditopic nitrogen-based acceptors.
New J. Chem., 2018,42, 10584-10591
https://doi.org/10.1039/C8NJ01368C
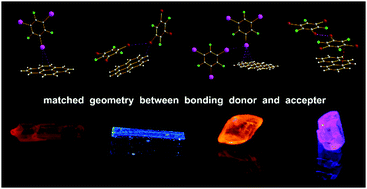
Cooperative halogen bonding and polarized π-stacking in the formation of coloured charge-transfer co-crystals
Matched electron rich halogen bond acceptors and donor have been synthesized and the halogen bonded charge transfer cocrystals characterized.
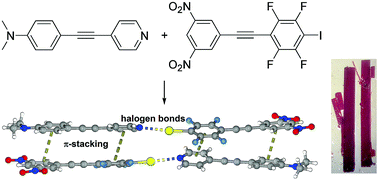
New J. Chem., 2018,42, 10615-10622
https://doi.org/10.1039/C8NJ00693H
May halogen bonding interactions compete with Cu⋯Cl semi-coordinate bonds? Structural, magnetic and theoretical studies of two polymorphs of trans-bis(5-bromo-2-chloro pyridine)dichlorocopper(II) and trans-bis(2,5-dichloropyridine)dichlorocopper(II)
Interaction of the negative potential area from one molecule with the positive areas I and II from two different molecules produces polymorphs 1 and 2.
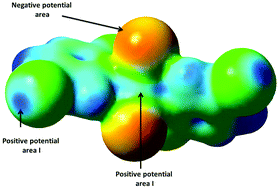
New J. Chem., 2018,42, 10642-10650
https://doi.org/10.1039/C8NJ00422F
Halogen bonding from the bonding perspective with considerations for mechanisms of thyroid hormone activation and inhibition
Bonding models of halogen bonding help understand how thyroid hormones and xenobiotic inhibitors affect thyroid activity through iodothyronine deiodinase.

New J. Chem., 2018,42, 10623-10632
https://doi.org/10.1039/C8NJ00557E
Cooperative role of halogen and hydrogen bonding in the stabilization of water adducts with apolar molecules
Conversely to the H2O–CF4 adduct, an appreciable intermolecular bond stabilization by charge transfer is operative in the H2O–CCl4 system.
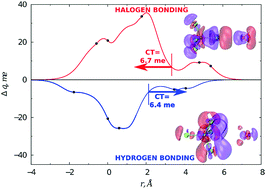
New J. Chem., 2018,42, 10603-10614
https://doi.org/10.1039/C8NJ00552D
Mechanochemistry and cocrystallization of 3-iodoethynylbenzoic acid with nitrogen-containing heterocycles: concurrent halogen and hydrogen bonding
Halogen-bonded and hydrogen-bonded cocrystals of 3-iodoethynylbenzoic acid and several nitrogen-containing heterocycles are formed using mechanochemical and solvent-based slow evaporation methods.
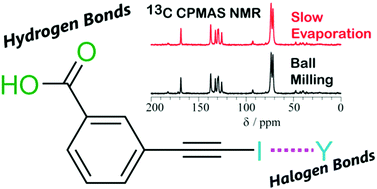
New J. Chem., 2018,42, 10493-10501
https://doi.org/10.1039/C8NJ00437D
Structural diversity in the products formed by the reactions of 2-arylselanyl pyridine derivatives and dihalogens
The presence of competing donor sites in L1–L4 influences their reactivity towards dihalogens and interhalogens.
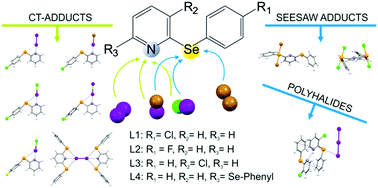
New J. Chem., 2018,42, 10592-10602
https://doi.org/10.1039/C8NJ00495A
Chalcogen bonding interactions in organic selenocyanates: from cooperativity to chelation
Organic selenocyanates form recurrent chain-like motifs ⋯Se(R)–CN⋯Se(R)–CN⋯ through short and linear chalcogen bonding Se⋯N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C interactions. A chelating motif is also observed in a DMF solvate with two neighboring CH2–SeCN groups linked to the DMF oxygen atom.
C interactions. A chelating motif is also observed in a DMF solvate with two neighboring CH2–SeCN groups linked to the DMF oxygen atom.
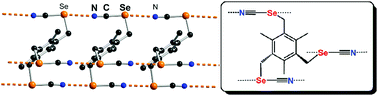
New J. Chem., 2018,42, 10502-10509
https://doi.org/10.1039/C8NJ00554K
Strengths of non-covalent interactions in hydrogen-bonded complexes B⋯HX and halogen-bonded complexes B⋯XY (X, Y = F, Cl): an ab initio investigation
The intermolecular quadratic stretching force constants kcalc.σ of a series of hydrogen-bonded and halogen bonded complexes B⋯HX and B⋯XY, where B is N2, CO, HCCH, C2H4, H2S, PH3, HCN, or NH3.
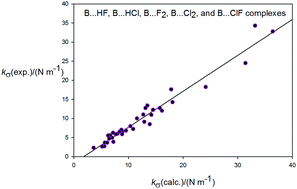
New J. Chem., 2018,42, 10548-10554
https://doi.org/10.1039/C8NJ00470F
Quantum calculations of At-mediated halogen bonds: on the influence of relativistic effects
If astatine is generally a stronger halogen-bond donor than iodine, an inversion is sometimes observed owing to the spin–orbit coupling.
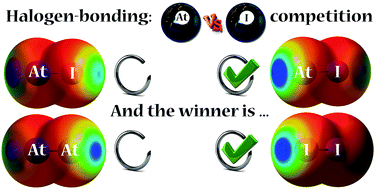
New J. Chem., 2018,42, 10510-10517
https://doi.org/10.1039/C8NJ00484F
Structural preferences in strong anion–π and halogen-bonded complexes: π- and σ-holes vs. frontier orbitals interaction
Intermolecular contacts in strong anion–π and halogen-bonded complexes follow frontier orbitals (instead of most positive or negative surface potentials) of reactants.
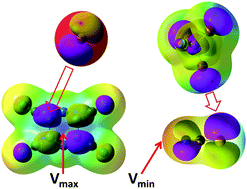
New J. Chem., 2018,42, 10572-10583
https://doi.org/10.1039/C7NJ04843B
Effect of geometry factors on the priority of σ-hole⋯π and π-hole⋯π bond in phosphorescent cocrystals formed by pyrene or phenanthrene and trihaloperfluorobenzenes
The effect of geometric factors on the assembly of luminescent cocrystals between pyrene or phenanthrene and trihaloperfluorobenzenes.
New J. Chem., 2018,42, 10633-10641
https://doi.org/10.1039/C7NJ04536K
Binding of alkyl halides in water-soluble cavitands with urea rims
Alkyl halide guests in cavitands move rapidly and maintain halide to contact with the aryl surfaces of the host.
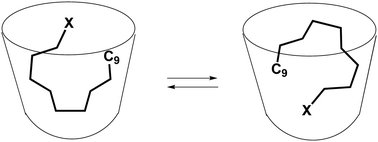
New J. Chem., 2018,42, 9945-9948
https://doi.org/10.1039/C8NJ01567H
About this collection
The halogen bond is the attractive interaction where the halogen atom functions as the electrophilic site. While the first halogen bonded adduct was synthesized more than two centuries ago, the understanding of the nature of this bond and the appreciation of its relevance in biosystems and materials is a much more recent story and experienced an exponential acceleration in the last decade. This themed issue in New Journal of Chemistry is the successful result of our aim to pursue a themed issue on halogen bonding in a broad-based primary journal encompassing all branches of chemistry and its sub-disciplines. This issue is particularly timely as there is a nice synchronization with the Third International Symposium on Halogen Bonding (ISXB-3; Greenville, SC, USA; 10th-14th June 2018), a major event for the community of those interested in the interaction.
The halogen bond had a seminal role in rationalizing the ability of the elements of Groups 14-18 of the periodic table to form the analogous chalcogen, pnictogen and tetrel bonds. Some papers of this themed issue discuss these new interactions, confirming how forward-looking this issue is. The synchronization mentioned above continues: an IUPAC sponsored workshop covering these interactions (IUPAC Workshop@ISXB-3) takes place prior to ISXB-3 at the same venue (Greenville; 9th-10th June).
Giuseppe Resnati and William T. Pennington, Guest Editors