Themed collection Carboranes

Front cover

Inside front cover

Carboranes
Welcome to this issue of Dalton Transactions featuring the themed issue on Carboranes.
Dalton Trans., 2014,43, 4924-4924
https://doi.org/10.1039/C4DT90026J
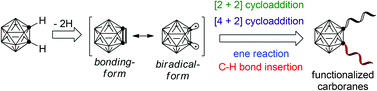
Generation and reactivity of o-carborynes
o-Carboryne is a very reactive intermediate and exists in two resonance forms that exhibit significantly different reactivity patterns, leading to the synthesis of a large class of functionalized o-carboranes.
Dalton Trans., 2014,43, 4925-4934
https://doi.org/10.1039/C3DT52711E
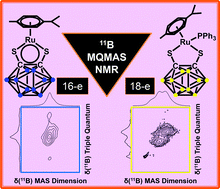
A multinuclear 1H, 13C and 11B solid-state MAS NMR study of 16- and 18-electron organometallic ruthenium and osmium carborane complexes
The first 1H, 13C, 31P and 11B solid state MAS NMR studies of electron-deficient carborane-containing ruthenium and osmium complexes [Ru/Os(p-cym)(1,2-dicarba-closo-dodecaborane-1,2-dithiolate)] are reported.
Dalton Trans., 2014,43, 4945-4949
https://doi.org/10.1039/C3DT53589D
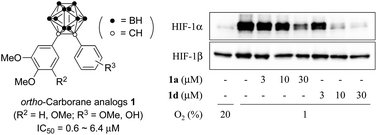
Diaryl-substituted ortho-carboranes as a new class of hypoxia inducible factor-1α inhibitors
Diaryl-substituted ortho-carboranes 1a and 1b, synthesized from the corresponding alkynes by decaborane coupling, inhibited HIF-1α accumulation without affecting HIF-1α mRNA expression under hypoxia.
Dalton Trans., 2014,43, 4941-4944
https://doi.org/10.1039/C3DT52828F
Reduction of hydroxy-functionalised carbaboranyl carboxylic acids to tertiary alcohols by organolithium reagents
Reduction of hydroxy-functionalised carbaboranyl carboxylic acids by organolithium reagents yields tertiary alcohols, while reaction of unsubstituted carbaboranyl carboxylic acids leads to cleavage of the exo-polyhedral C–C bond.
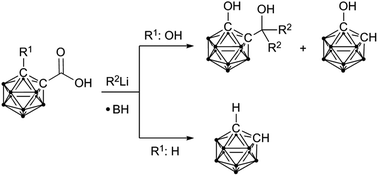
Dalton Trans., 2014,43, 4935-4937
https://doi.org/10.1039/C3DT52826J
Nucleophilic addition of carborane anion to Ir, Rh-coordinated Cp* ring: C–C bond formation accompanied by reduction of metal center
A C–C bond formation complex and a Cl−/Nu− exchange complex have been obtained through nucleophilic addition by using different nucleophiles.
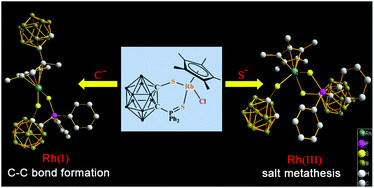
Dalton Trans., 2014,43, 4938-4940
https://doi.org/10.1039/C3DT51906F
Preparation and evaluation of carborane-derived inhibitors of prostate specific membrane antigen (PSMA)
Carborane-derived inhibitors of prostate specific membrane antigen are reported. Compounds were prepared from C-hydroxy-carboranes and screened in vitro and in vivo.
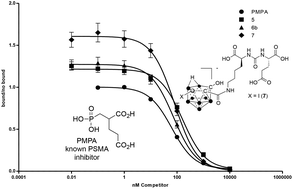
Dalton Trans., 2014,43, 4950-4961
https://doi.org/10.1039/C3DT53189A
Base-mediated transformation of the agostic (π-allyl)-closo-rhodacarboranes into complexes with an open (1-5-η5)-pentadienyl ligand
Base-mediated linear coupling reactions of the agostic (CH3⋯Rh) (π-allyl)rhodacarborane complexes afforded the structurally novel mononuclear species bearing an open (1-5-η5)-pentadienyl ligand at the metal vertex.
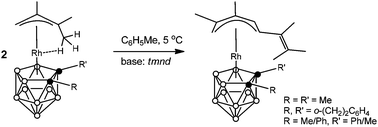
Dalton Trans., 2014,43, 5076-5082
https://doi.org/10.1039/C3DT52838C
Switchable NLO response induced by rotation of metallacarboranes [NiIII/IV(C2B9H11)2]−/0 and C-,B-functionalized derivatives
Geometric interconversions of metallacarboranes and the derivatives resulting from the redox reaction allow the NLO responses to be switched “ON” or “OFF”.
![Graphical abstract: Switchable NLO response induced by rotation of metallacarboranes [NiIII/IV(C2B9H11)2]−/0 and C-,B-functionalized derivatives](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3DT53298D&imageInfo.ImageIdentifier.Year=2014)
Dalton Trans., 2014,43, 5069-5075
https://doi.org/10.1039/C3DT53298D
Synthesis, characterisation and some chemistry of C- and B-substituted carboxylic acids of cobalt bis(dicarbollide)
Active esters of cobalt bis(dicarbollide)(1−) carboxylic acids allow for incorporation of this ion into functional molecules by amidic bonds.
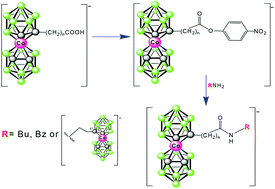
Dalton Trans., 2014,43, 5106-5120
https://doi.org/10.1039/C3DT52870G
Syntheses and structural characterization of o-carboranylamides with direct cage–amide bond
Reactions of lithio-o-carborane with isocyanates give carboranylamides in which the substituent and solvent effects influence the product distribution and yields.
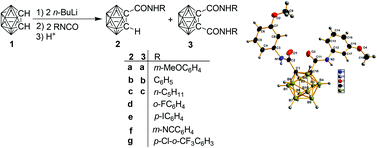
Dalton Trans., 2014,43, 5083-5094
https://doi.org/10.1039/C3DT52785A
Surfactant behaviour of metallacarboranes. A study based on the electrolysis of water
Cobaltabisdicarbollide and its chloroderivatives lack the amphiphilic topology but behave as anionic surfactants in water electrolysis. The worst performing as electrolyte is pristine [3,3′-Co(C2B9H11)2]−, whilst the best is [B12H12]2−; their difference being the capacity to generate self-assembling ordering by B–H⋯H–Cc dihydrogen bonds.
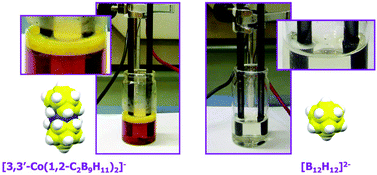
Dalton Trans., 2014,43, 5062-5068
https://doi.org/10.1039/C3DT52825A
Preparation and characterization of Au nanoparticles capped with mercaptocarboranyl clusters
High boron content gold nanoparticles exhibit complete dispersibility in water, opening the way for their use in medical applications.
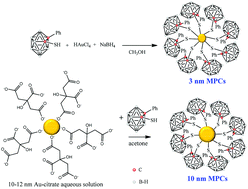
Dalton Trans., 2014,43, 5054-5061
https://doi.org/10.1039/C3DT52872C
A new approach to the synthesis of functional derivatives of nido-carborane: alkylation of [9-MeS-nido-7,8-C2B9H11]−
A series of diastereomeric sulfonium derivatives of nido-carborane [9-R(Me)S-nido-7,8-C2B9H11] were prepared for the first time by alkylation of 9-methylthio-nido-7,8-carborane [9-MeS-nido-7,8-C2B9H11]−.
![Graphical abstract: A new approach to the synthesis of functional derivatives of nido-carborane: alkylation of [9-MeS-nido-7,8-C2B9H11]−](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3DT52845F&imageInfo.ImageIdentifier.Year=2014)
Dalton Trans., 2014,43, 5044-5053
https://doi.org/10.1039/C3DT52845F
1,3,2-Dithiaphospholanes with an annelated 1,2-dicarba-closo-dodecaborane(12) unit: formation and reactivity
Some 1,3,2-dithiaphospholanes with P-organo substituents dimerise reversibly, except R = t-Bu, as shown by NMR, DFT calculations and X-ray analysis.
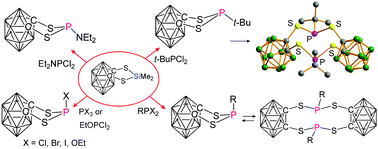
Dalton Trans., 2014,43, 5021-5043
https://doi.org/10.1039/C3DT52753K
Novel inorganic heterocycles from dimetalated carboranylamidinates
An efficient synthetic route to novel inorganic ring systems based on dianionic carboranylamidinates is reported.
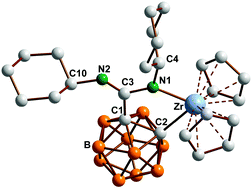
Dalton Trans., 2014,43, 5001-5013
https://doi.org/10.1039/C3DT52751D
Synthesis and structural characterization of 14-vertex germa-, stanna-, and plumba-carboranes
14-Vertex germa-, stanna-, and plumba-carboranes have been prepared and structurally characterized via polyhedral expansion methodology, which represents the first examples of p-block metallacarboranes of the MC2B11 system.
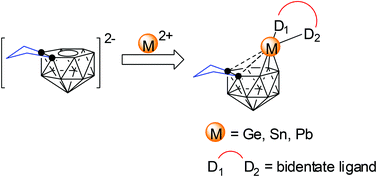
Dalton Trans., 2014,43, 4986-4992
https://doi.org/10.1039/C3DT52406J
Cross-coupling reaction between arylboronic acids and carboranyl iodides catalyzed by graphene oxide (GO)-supported Pd(0) recyclable nanoparticles for the synthesis of carboranylaryl ketones
Synthesis of carboranylaryl ketones by carbonylative cross-coupling reactions catalyzed by Pd@GOs.
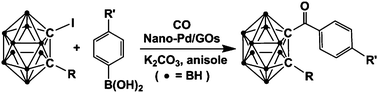
Dalton Trans., 2014,43, 5014-5020
https://doi.org/10.1039/C3DT52560K
Synthesis of closo- and nido-biscarboranes with rigid unsaturated linkers as precursors to linear metallacarborane-based molecular rods
A number of biscarborane precursors to linear metallacarborane-based molecular rods were prepared from 8-iodo-1,2-dicarba-closo-dodecaborane.

Dalton Trans., 2014,43, 4969-4977
https://doi.org/10.1039/C3DT52596A
Through-space charge transfer and emission color tuning of di-o-carborane substituted benzene
The electronic variation of the appended aryl group at the C1-position of o-carborane in the di-o-carborane substituted benzene compounds led to color tuning of aggregation-induced emission.
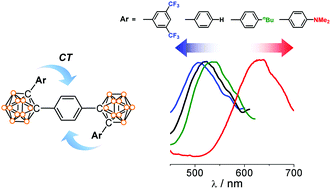
Dalton Trans., 2014,43, 4978-4985
https://doi.org/10.1039/C3DT52465E
Asymmetric 1,8/13,2,x-M2C2B10 14-vertex metallacarboranes by direct electrophilic insertion reactions; the VCD and BHD methods in critical analysis of cage C atom positions
Bicapped hexagonal antiprismatic bimetallacarboranes with 1,8/13,2,x-Co2C2B10 architectures have been characterised including cage C atom identification in crystallographic studies.

Dalton Trans., 2014,43, 5095-5105
https://doi.org/10.1039/C3DT52101J
Deltahedral ferratricarbaboranes: analogues of ferrocene
The lowest energy CpFeC3Bn−4Hn−1 (n = 8 to 12) structures are found to have the maximum number of carbon atoms at degree 4 rather than degree 5 vertices and avoid adjacent carbon atoms, i.e., C–C edges. Hexagonal bipyramidal structures are found for CpFeC3B4H7, isocloso structures are found for CpFeC3Bn−4Hn−1 (n = 8, 9), and polyhedral structures with quadrilateral faces are found for CpFeC3Bn−4Hn−1 (n = 10, 11).
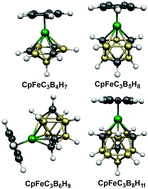
Dalton Trans., 2014,43, 4993-5000
https://doi.org/10.1039/C3DT52381K
Unusual cationic rhodathiaboranes: synthesis and characterization of [8,8,8-(H)(PR3)2-9-(Py)-nido-8,7-RhSB9H10]+ and [1,3-μ-(H)-1,1-(PR3)2-3-(Py)-isonido-1,2-RhSB9H8]+
A unique set of cationic polyhedral boron-based clusters is reported to exhibit remarkable structural non-rigidity with potential in bond activation.
![Graphical abstract: Unusual cationic rhodathiaboranes: synthesis and characterization of [8,8,8-(H)(PR3)2-9-(Py)-nido-8,7-RhSB9H10]+ and [1,3-μ-(H)-1,1-(PR3)2-3-(Py)-isonido-1,2-RhSB9H8]+](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3DT52018H&imageInfo.ImageIdentifier.Year=2014)
Dalton Trans., 2014,43, 5121-5133
https://doi.org/10.1039/C3DT52018H
Radical coupling for directed C–C/C–S bond formation in the reaction of Cp*IrS2C2B10H10 with 1-azido-3-nitrobenzene
The reaction of Cp*IrS2C2B10H10 (1) with 1-azido-3-nitrobenzene affords 2–6 with C–C and C–S bond formation via a radical mechanism.
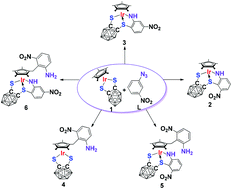
Dalton Trans., 2014,43, 4962-4968
https://doi.org/10.1039/C3DT52308J
About this collection
This themed issue focuses on the chemistry and application of carboranes and was guest edited by Professors Guo-Xin Jin and Zuowei Xie.