1,3,2-Dithiaphospholanes with an annelated 1,2-dicarba-closo-dodecaborane(12) unit: formation and reactivity†
Abstract
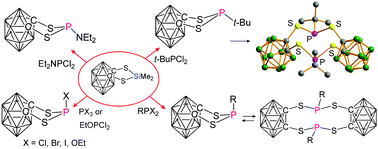
1,3,2-Dithiaphospholanes were prepared, containing an annelated 1,2-dicarba-closo-dodecaborane(12) unit, bearing halogens (F, Cl, Br, and I), H, NEt2, OEt groups, or organo substituents (R = i-Pr, t-Bu, Cy, 3,5-Me2-C6H3-CH2, Ph) at phosphorus. The t-Bu derivative was structurally characterised as its sulfide. The organo-substituted derivatives escape the ring strain by irreversible (R = t-Bu; X-ray analysis) or reversible dimerisation, by which 10-membered rings are formed. Using 1,1-bis(dichlorophosphino)methane, another dimer containing a 14-membered ring could be isolated and structurally characterised. The preferred conformation of the 1,3,2-dithiaphospholanes depends on the nature of the substituent at phosphorus. For instance, the substituents R = t-Bu, NEt2 prefer the equatorial position, as indicated by diagnostic NMR data, supported by DFT calculations [B3LYP/6-311+G(d,p) level of theory]. The calculations also predict the tendency for dimerisation, and calculated NMR parameters are in good agreement, in most cases, with experimental data.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...