Highly regio- and stereoselective trans-iodofluorination of ynamides enabling the synthesis of (E)-α-fluoro-β-iodoenamides†
Abstract
A highly regio- and stereoselective trans-iodofluorination reaction of ynamides with NIS and Et3N·3HF has been achieved, affording (E)-α-fluoro-β-iodoenamides in moderate to good yields. The reaction proceeds under mild reaction conditions and exhibits good functional group compatibility.
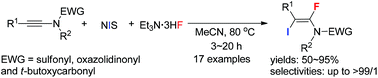


 Please wait while we load your content...
Please wait while we load your content...