2,4-Diamino-1,3,5-triazine-enabled Cu-catalyzed direct sulfonamidation of aromatic C–H bonds†
Abstract
An efficient copper acetate catalyzed sulfonamidation of arenes via C–H bond activation directed by a 2,4-diamino-1,3,5-triazine chelating group under oxygen as a terminal oxidant has been developed. The reaction shows good regioselectivity and functional group tolerance, as well as providing a straightforward methodology for the preparation of ortho-monosulfonamidated arene derivatives in moderate to high yields. The sulfonamidation at the gram scale can be performed with a good yield.
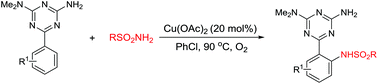


 Please wait while we load your content...
Please wait while we load your content...