Palladium-catalyzed allylic amination: a powerful tool for the enantioselective synthesis of acyclic nucleoside phosphonates†
Abstract
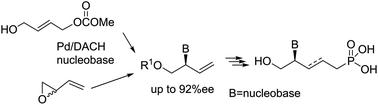
Acyclic nucleoside phosphonates have been prepared in a straightforward manner and in high yields by an enantioselective palladium-catalyzed allylic substitution involving nucleic bases as nucleophiles followed by cross-metathesis reaction with diethyl allylphosphonate.


 Please wait while we load your content...
Please wait while we load your content...