Investigation of benzoyloximes as benzoylating reagents: benzoyl-Oxyma as a selective benzoylating reagent†
Abstract
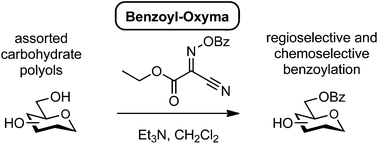
Hydroxybenzotriazole (HOBt) and HOBt-derived reagents have been classified as Class I explosives, with restrictions on their transportation and storage. We explored a range of benzoylated oxime-based reagents as alternatives to benzoyloxybenzotriazole (BBTZ) for the selective benzoylation of carbohydrate polyols. Benzoylated oximes derived from 2-hydroximino-malononitrile, ethyl 2-hydroximino-2-cyanoacetate (Oxyma), and tert-butyl 2-hydroximino-2-cyanoacetate were most effective for benzoylation of a simple primary alcohol, with yields approaching that obtained for BBTZ. When applied to carbohydrate diols, the most effective reagent was identified as benzoyl-Oxyma. Benzoyl-Oxyma is a highly crystalline, readily prepared alternative to BBTZ, useful in the selective benzoylation of carbohydrate polyols.

 Please wait while we load your content...
Please wait while we load your content...