Evaluation of fluoropyruvate as nucleophile in reactions catalysed by N-acetyl neuraminic acid lyase variants: scope, limitations and stereoselectivity†
Abstract
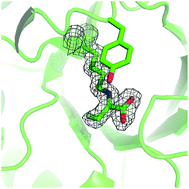
The catalysis of reactions involving fluoropyruvate as donor by N-acetyl neuraminic acid lyase (NAL) variants was investigated. Under kinetic control, the wild-type enzyme catalysed the reaction between fluoropyruvate and N-acetyl mannosamine to give a 90 : 10 ratio of the (3R,4R)- and (3S,4R)-configured products; after extended reaction times, equilibration occurred to give a 30 : 70 mixture of these products. The efficiency and stereoselectivity of reactions of a range of substrates catalysed by the E192N, E192N/T167V/S208V and E192N/T167G NAL variants were also studied. Using fluoropyruvate and (2R,3S)- or (2S,3R)-2,3-dihydroxy-4-oxo-N,N-dipropylbutanamide as substrates, it was possible to obtain three of the four possible diastereomeric products; for each product, the ratio of anomeric and pyranose/furanose forms was determined. The crystal structure of S. aureus NAL in complex with fluoropyruvate was determined, assisting rationalisation of the stereochemical outcome of C–C bond formation.

 Please wait while we load your content...
Please wait while we load your content...