Nitrite ion sensing properties of ZnTiO3–TiO2 composite thin films deposited from a zinc–titanium molecular complex†
Abstract
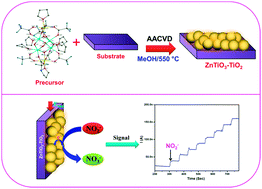
A titanium based heterobimetallic molecular precursor, [Zn2Ti4(μ-O)6(TFA)8(THF)6]·THF (1) (where TFA = trifluoroacetato; THF = tetrahydrofuran), has been designed and scrutinised for its various physicochemical properties by melting point analysis, microanalysis, Fourier transform infra-red spectroscopy, proton nuclear magnetic resonance spectroscopy, thermogravimetry and single crystal X-ray structural analysis. ZnTiO3–TiO2 composite thin films were grown on a fluorine doped tin oxide (FTO) coated conducting glass substrate at 550 °C from three different solutions of (1) viz. methanol, THF and acetonitrile, by the aerosol-assisted chemical vapour deposition technique. The phase identification, chemical composition and microstructure of the fabricated thin films that were probed by powder X-ray diffraction, Raman spectroscopy, energy dispersive X-ray analysis and scanning electron microscopy revealed the formation of a 1 : 1 ratio of ZnTiO3 : TiO2 composite microspheres of diverse designs and textures depending on the type of deposition solvent used. The direct band gap energy of 3.1 eV was estimated by UV-visible spectrophotometry of the ZnTiO3–TiO2 film fabricated from methanol solution and the film electrode was further tested as an electrochemical sensor for the detection of nitrite ions.

 Please wait while we load your content...
Please wait while we load your content...