Probing the effect of arm length and inter- and intramolecular interactions in the formation of Cu(ii) complexes of Schiff base ligands derived from some unsymmetrical tripodal amines†
Abstract
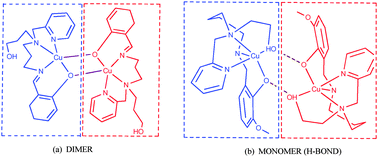
The syntheses of two previously known, 2-((2-aminoethyl)(pyridin-2-ylmethyl)amino)ethanol (1) and 2-((3-aminopropyl)(pyridin-2-ylmethyl)amino)ethanol (2), and four new unsymmetrical N-capped tripodal amines, 2-((4-aminobutyl)(pyridin-2-ylmethyl)amino)ethanol (3), 3-((2-aminoethyl)(pyridin-2-ylmethyl)amino)propan-1-ol (4), 3-((3-aminopropyl)(pyridin-2-ylmethyl)amino)propan-1-ol (5) and 3-((4-aminobutyl)(pyridin-2-ylmethyl)amino)propan-1-ol (6), are reported. The ligands (3–4) feature a longer arm, 3-hydroxypropyl or butylamino, than in the analogues previously employed (2-hydroxyethyl arm, ethylamino-arm or propylamino-arm in 1 and 2). All six tripodal amines, 1–6, are equipped with a 2-methylpyridyl-arm and either an ethylamino-arm (1 and 4), propylamino-arm (2 and 5) or butylamino-arm (3 and 6). The new amines, 3–6, have been employed in one pot condensation reactions with 2-hydroxy-1-naphthaldehyde and salicylaldehyde (and its derivatives) in the presence of Cu(II) metal ions to generate a series of new mononuclear complexes, [MIILaldi](ClO4) as well as new dinuclear complexes [CuIILaldi]2(ClO4)2 of new ligands Laldi. Four monomeric complexes and one dimeric complex have been characterised by single crystal X-ray diffraction, revealing a distorted square-pyramidal copper(II) ion. A general comparison between these structures shows that the number and types of chelate ring sequences around the metal ions are important in the formation of structures. Theoretical studies show that the 3-hydroxypropyl arm in these complexes is a weak coordinating group and it can readily be removed from the coordination sphere of metal ions, resulting in a dimerised four coordinate complex. Calculations show that the interaction between the two monomeric fragments is very weak.

 Please wait while we load your content...
Please wait while we load your content...