On the energetics of cation ordering in tungsten-bronze-type oxides†
Abstract
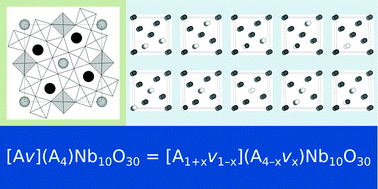
Oxides with the tetragonal tungsten bronze (TTB) structure are well-known ferroelectrics that show a large flexibility both with respect to chemical composition and cation ordering. Two of the simplest compounds in this family are lead metaniobate (PbNb2O6 or PN) and strontium barium niobate (SrxBa1−xNb2O6 or SBN). While PN is a classical ferroelectric, SBN goes from ferroelectric to relaxor-like with increasing Sr content, with a polar direction different from that in PN. The partially occupied sublattices in both systems give the possibility for cation order–disorder phenomena, but it is not known if or how this influences the polarization and ferroelectricity. Here, we use density functional theory (DFT) calculations to investigate how cation and cation vacancy ordering influences the energetics of these compounds, by comparing both the energy differences and the barriers for transition between different cation configurations. We extend the thermodynamic model of O'Neill and Navrotsky, originally developed for cation interchange in spinels, to describe the order–disorder phenomenology in TTB oxides. The influence of order–disorder processes on the functional properties of PN and SBN is discussed.

 Please wait while we load your content...
Please wait while we load your content...