Solubility of n-butane and 2-methylpropane (isobutane) in 1-alkyl-3-methylimidazolium-based ionic liquids with linear and branched alkyl side-chains†
Abstract
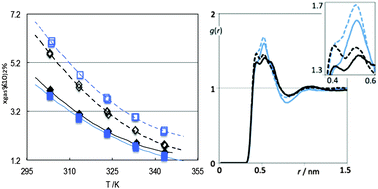
The solubility of n-butane and 2-methylpropane (isobutane) in three ionic liquids – 1-(2-methylpropyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [(2mC3)C1im][Ntf2], 1-(3-methylbutyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [(3mC4)C1im][Ntf2] and 1-methyl-3-pentylimidazolium bis(trifluoromethylsulfonyl)imide [C5C1im][Ntf2] – has been measured at atmospheric pressure from 303 to 343 K. Isobutane is less soluble than n-butane in all the ionic liquids. Henry's constant values range from 13.8 × 105 Pa for n-butane in [C5C1im][Ntf2] at 303 K to 64.5 × 105 Pa for isobutane in [(2mC3)C1im][Ntf2] at 343 K. The difference in solubility between the two gases can be explained by a more negative enthalpy of solvation for n-butane. A structural analysis of the pure solvents and of the solutions of the gases, probed by molecular dynamics simulations, could explain the differences found in the systems: (i) the nonpolar domains of the ionic liquids accommodate better the long and more flexible n-butane solute; (ii) the small differences in solubility of each gas in the ionic liquids with the same number of carbon atoms in the alkyl side-chains are explained by the absence of large structural differences in the pure solvents. In all cases, the structural analysis of the four ionic liquids confirms that the studied gases can act as probes of the molecular structure of the ionic liquids, the simulations being always compatible with the experimental solubility data.

 Please wait while we load your content...
Please wait while we load your content...