Controlling charge separation in a novel donor–acceptor metal–organic framework via redox modulation†
Abstract
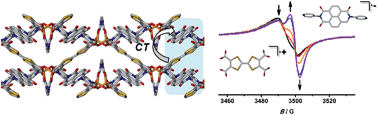
Charge transfer metal–organic frameworks represent a versatile class of multifunctional materials that offer an unprecedented combination of physical properties. The framework [(Zn(DMF))2(TTFTC)(DPNI)] incorporating the donor and acceptor, tetrathiafulvalenetetracarboxylate (TTFTC) and N,N′-di-(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide (DPNI) respectively, exhibits charge transfer by virtue of donor–acceptor interactions within its crystalline structure. This through-space interaction is manifested by the formation of ligand-based radicals in the as-synthesised material and leads to a partial degree of charge separation. Five distinct electronic states of the framework can be accessed using solid state electrochemical and spectroelectrochemical techniques, including for the first time in application to metal–organic frameworks, EPR spectroelectrochemistry (SEC). The degree of charge transfer is controllable via redox modulation and has been quantified using complementary DFT modelling of the charge transfer states.

 Please wait while we load your content...
Please wait while we load your content...