Heterolytic cleavage of H2 by bifunctional manganese(i) complexes: impact of ligand dynamics, electrophilicity, and base positioning†
Abstract
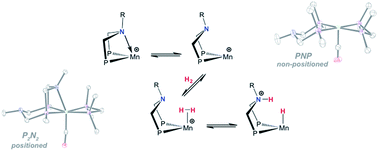
We report the synthesis, characterization, and reactivity with H2 of a series of MnI complexes of the type [(P–P)Mn(L2)CO]+ (L2 = dppm, bppm, or (CO)2; P–P = PPhNMePPh or PPh2NBn2) that bear pendant amine ligands designed to function as proton relays. The pendant amine was found to function as a hemilabile ligand; its binding strength is strongly affected by the ancillary ligand environment around Mn. Tuning the electrophilicity of the Mn center leads to systems capable of reversible heterolytic cleavage of the H–H bond. The strength of pendant amine binding can be balanced to protect the Mn center while still leading to facile reactivity with H2. Neutral MnIH species bearing pendant amines in the diphosphine ligand were found to react with one-electron oxidants and, after proton and electron transfer reactions, regenerate cationic MnI species. The reactivity presented herein indicates that the Mn complexes we have developed are a promising platform for development of Mn-based H2 oxidation electrocatalysts.

 Please wait while we load your content...
Please wait while we load your content...