Improved visible-light activities of nanocrystalline CdS by coupling with ultrafine NbN with lattice matching for hydrogen evolution†
Yang
Qu
a,
Ning
Sun
a,
Muhammad
Humayun
a,
Amir
Zada
a,
Ying
Xie
a,
Junwang
Tang
b,
Liqiang
Jing
 *a and
Honggang
Fu
*a and
Honggang
Fu
 *a
*a
aKey Laboratory of Functional Inorganic Materials Chemistry (Heilongjiang University), Ministry of Education, School of Chemistry and Materials Science, International Joint Research Center for Catalytic Technology, Harbin 150080, P. R. China. E-mail: jinglq@hlju.edu.cn; fuhg@hlju.edu.cn
bDepartment of Chemical Engineering, University College London, Torrington Place, London WC1E 7JE, UK. E-mail: Junwang.tang@ucl.ac.uk
First published on 22nd January 2018
Abstract
Metallic ultrafine NbN with lattice matching to nanocrystalline CdS could replace the expensive platinum for efficiently trapping photogenerated electrons, enhancing charge separation and then activating the preferentially adsorbed H2O to produce H2, mainly confirmed by Kelvin probe experiments, Mott–Schottky plots, amount of ˙OH produced, electrochemical experiments and density-functional-theory calculations.
Semiconductor-based photocatalytic hydrogen production using solar energy has been considered to be one of the most significant approaches to solve the worldwide energy crisis.1–3 It is widely accepted that semiconductor materials play a vital role in photocatalysis. An ideal semiconductor photocatalyst has to possess broad-spectrum light harvesting, effective charge separation and efficient surface catalytic/active capacity. Over the past few decades, great attention has been focused toward the synthesis of efficient photocatalysts and the study of photocatalytic mechanisms.4–6 However, the efficiencies of photocatalytic hydrogen production for state-of-the-art devices are still unsatisfactory for industry due to several shortcomings of the semiconductor photocatalysts, especially the sluggish charge separation and poor surface activation.7–10
CdS with a suitable electronic bandgap (Eg = 2.4 eV, with the conduction band at −0.52 eV vs. NHE) is an ideal semiconductor for photocatalytic H2 evolution.11 However, shortcomings such as sluggish charge separation and poor surface activation of H2O result in poor photocatalytic activity for H2 production.12 It is therefore much meaningful to synchronously enhance the charge separation and improve the surface activation of H2O so as to promote the photocatalytic activity of CdS. Usually, platinum (Pt) is widely used as a co-catalyst to enhance the charge separation through the formed Schottky barrier and improve the surface catalytic capacity via its natural catalytic ability.4,13,14 However, the high cost and resource shortage limit the practical applications of Pt. Therefore, new substituents are urgently needed. Although some metallic transition metal sulfides and carbides, such as WS2, MoS2, MoB2 and WC, have been tried, the H2 production activities are still unsatisfactory owing to the poor charge transfer across the interface of mismatched lattices and weak adsorption of H2O on their surfaces.15–18 As a consequence, new materials that show lattice matching with CdS are still required. Emphatically, the lattice matching of two different components in heterojunctions is very important for charge separation.19,20
Niobium nitride (NbN) is a well-known metallic superconductor and has been considered to be one of the promising cryoelectronic materials.21 Significantly, it has similar crystal lattices to that of CdS, along with perfectly matched lattices. As shown in Fig. S1,† density functional theory (DFT) reveals a mere 0.03% mismatch between the (002) lattice planes of NbN and CdS, which is much better than that of the (002) planes of Pt/CdS (1.5% mismatch). Moreover, NbN is reported to be metallic with a low work function, suggesting that it is a rational candidate to improve the photocatalytic activity of CdS for H2 production.22 To date, studies focusing on NbN/CdS nanocomposites for photocatalysis are scarce.
Hence, we report an interesting NbN/CdS nanocomposite for photocatalytic hydrogen production under visible light irradiation. Nanocrystalline NbN was synthesized by an ammonolysis method. CdS nanocrystallites were grown in the presence of NbN via a chemical bath deposition method to prepare NbN/CdS nanocomposites (see the ESI†). The XRD pattern in Fig. S2† shows the five Bragg diffraction peaks of bulk NbN which are in good agreement with the standard card (JCPDS no. 89-6042, a = b = 4.107027 Å).23 Raman spectra (Fig. S3†) also confirm the preparation of pure-phase NbN.24 The diffraction peaks of the bare nanocrystalline CdS are well matched with the standard card (JCPDS no. 41-1049, a = b = 4.10829 Å). XRD and Raman spectra demonstrate the successful preparation of NbN/CdS nanocomposites.
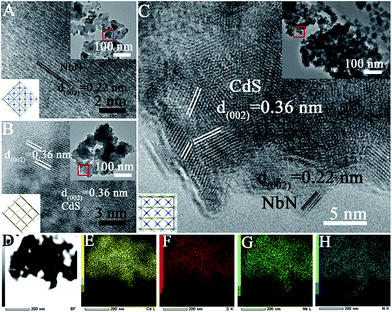
The SEM image (Fig. S4†) and the TEM image in the inset of Fig. 1 A, demonstrate that the average diameter of the as-prepared ultrafine NbN nanocrystallite is approximately 30 nm. The HRTEM image (Fig. 1A) reveals that the lattice fringes with a d-spacing of 0.22 nm correspond to the (002) crystallographic planes of ultrafine NbN.
The average diameter of bulk nanocrystalline CdS is 5–10 nm as shown in Fig. 1B with serious aggregation (inset of Fig. 1B). The TEM image of NbN/CdS nanocomposites (NC-1) in the inset of Fig. 1C reveals that the dispersion of CdS nanocrystallites is improved after coupling with ultrafine NbN. The lattice fringe at the (002) plane with a d-spacing of 0.22 nm in the HRTEM image (Fig. 1C) corresponds to NbN while that at the (002) plane with a d-spacing of 0.36 nm is attributed to CdS. The TEM mapping of NC-1 (Fig. 1D) reveals that the nanocomposite is made up of contributions from each element, i.e. Cd (Fig. 1E), S (Fig. 1F), Nb (Fig. 1G) and N (Fig. 1H), exhibiting a high dispersion of ultrafine NbN and good contact between NbN and CdS in the nanocomposites. XPS in Fig. S5A† further demonstrates the chemical composition of CdS and NbN. The high resolution XPS (Fig. S5B and C†) shows that the peak position of S in the nanocomposite slightly shifts toward higher binding energy compared to the bare CdS while that of Nb shifts toward lower binding energy revealing that a heterojunction between NbN and CdS in the nanocomposite has been formed. The well matched lattices of (002) NbN and (002) CdS are favorable to the charge separation, leading to enhanced photocatalytic activity.
Fig. 2A shows the photocatalytic H2 evolution activities of various samples. The photocatalytic activity of the mechanically mixed sample MNC-1 further reveals that coupling of NbN is feasible. One can see that the photocatalytic activity of nanocrystalline CdS is obviously enhanced after coupling with a small amount of NbN. NC-1 exhibits optimal photocatalytic activity under visible light irradiation for 1 h, which is up to 126 μmol over 0.1 g catalyst. If the amount of NbN is increased continuously, its black color affects the light absorption of CdS (Fig. S6†), which results in lower activity. The time course H2 evolution activities (Fig. S7†) show that the samples are good and stable. Importantly, the photocatalytic activity of NC-1 is obviously 1.4 times higher than that of 1 wt% Pt–CdS (90 μmol over 0.1 g catalyst). Moreover, the apparent quantum efficiency (QE) of NC-1 (2.2%) is higher than that of 1 wt% Pt/CdS (1.5%) at 420 nm. Compared to Pt/CdS, NC-1 also exhibits long time stability for six successive photocatalytic cycles in 24 h, suggesting that the NbN/CdS nanocomposites of NC-1 are highly stable (Fig. 2B). The stability is further confirmed by measuring the concentration of Cd2+ in the solution after 12 h irradiation by atomic absorption spectrometry, as shown in Table S1.† From N2 adsorption–desorption isotherms (Fig. S8†), it is clear that the surface area hardly contributed to the enhanced photocatalytic activity. The improved photocatalytic activity of NC-1 could be attributed to the well matched lattices that enhance charge separation. A comparison of the photocatalytic activities in Fig. S9† clearly demonstrates that the lattice matching positively affects the photocatalytic activities.
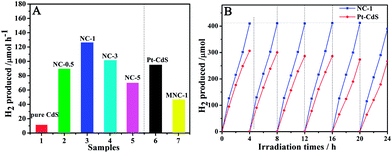 | ||
| Fig. 2 Photocatalytic H2 production activities of different samples (A) and stability of NC-1 and 1 wt% Pt/CdS in 24 h continuous photocatalytic cycles for H2 production (B). | ||
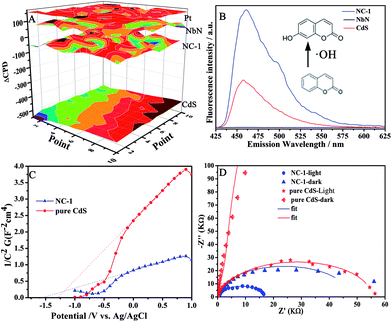
Besides, there are other reasons for the enhanced charge separation in NbN/CdS nanocomposites. DFT theoretical calculations of the partial density of states for NbN show all the electron orbits across the Fermi level plane (Fig. S10†), suggesting that NbN is metallic as it was reported.25 Additionally, the work functions of Pt, NbN, CdS, and NC-1 are about 5.25, 5.18, 4.6 and 5.06 eV, respectively, as measured from Kelvin probe experiments (Fig. 3A). The metallic NbN possesses a lower Fermi level than CdS, suggesting that it is an electron collector in the NbN/CdS heterojunction nanocomposite. Remarkably, the contact potential difference (CPD) of NbN/CdS is 70 mV smaller than that of Pt/CdS, implying that the lower energy barrier between CdS and NbN is very small for charge transfer.26 To further confirm the charge separation, the coumarin fluorescence method was used to detect the amount of hydroxyl radicals (˙OH) produced, in which coumarin could easily react with the formed ˙OH and produce luminescent 7-hydroxy-coumarin. As demonstrated previously, the ˙OH amount could effectively reveal the separation of photogenerated charges in the photocatalysis.27 As expected, it is confirmed from Fig. 3B that the ˙OH amount produced by NC-1 is much significant as compared to that produced by the bare CdS and NbN. Hence, it is deduced that the increase in the amount of coupled NbN is directly related to the enhanced charge separation.
The carrier density (Nd) of semiconductors is a significant parameter to measure the charge separation in heterojunctions.28Nd can be calculated from the slope of the Mott–Schottky plot using the following equation:29
| Nd = (2/e0εε0)[d(1/C2)/dV]−1 |
where e0 is the electron charge, ε is the dielectric constant, ε0 is the permittivity of vacuum, Nd is the carrier density and V is the applied bias at the electrode. It is thus the slope of the Mott–Schottky plot that reveals the carrier density, with an inversely proportional relationship. From Fig. 3C, NC-1 shows a smaller slope of the Mott–Schottky plot compared to the CdS nanoparticles, suggesting a high carrier density at the contact interface. The smaller arc, which belongs to NC-1 in the middle frequency region of the electrochemical impedance spectra (EIS) in Fig. 3D, demonstrates that the interfacial resistance becomes much lower after coupling NbN with CdS, favoring charge transfer and separation. The Mott–Schottky plot and EIS confirm that the interface of NbN and CdS favors charge transfer and separation. This can be further supported by the photoluminescence spectra in Fig. S11.†
Following charge separation, the adsorption and activation of H2O on the surface of the photocatalyst are much crucial for the photocatalytic activity of H2 reduction.30,31 However, the surface photochemical mechanism of NbN is so far unclear and rarely investigated previously. With regard to this, the adsorption and activation of H2O on the surface of the optimal NbN/CdS nanocomposite were studied. Theoretical calculations via DFT, in the inset of Fig. 4, show that the adsorption energy of the water molecule on (002) NbN is −1.8 eV, while it is −0.5 eV for (002) Pt, demonstrating that NbN possesses strong adsorption for H2O on its surface. Thus, H2O is more favorably adsorbed on NbN revealing that NbN is appropriate for photocatalytic hydrogen production, in particular after coupling with lattice-matched CdS. Further, electrochemical water reduction was carried out as shown in Fig. 4. The reductive current of NC-1 is −0.76 mA cm−2 which is much closer to that of 1 wt% Pt/CdS (−0.78 mA cm−2) at the potential of −1.2 V (Ag/AgCl). These electrochemical properties reveal that NbN could act as a co-catalyst to effectively catalyze hydrogen production by the collected photogenerated electrons.
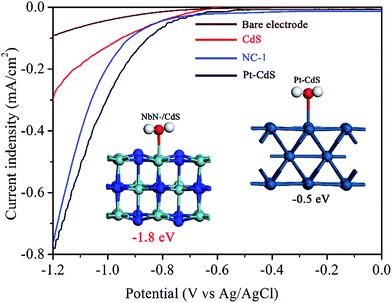 | ||
| Fig. 4 Electrochemical hydrogen reduction curves of the bare electrode, CdS, NC-1 and Pt/CdS with the inset showing the adsorption energies of H2O on (002) NbN and (002) Pt. | ||
In summary, NbN/CdS nanocomposites were prepared for high efficiency photocatalytic hydrogen production which was 10 times and 1.4 times higher than that of bulk CdS and Pt/CdS respectively, and displayed high stability even after six successive photocatalytic cycles. It was demonstrated that the enhanced photocatalytic activity could be attributed to the lattice matching and the heterojunctions formed between metallic NbN and CdS. Emphatically, in this work, NbN as a new type of co-catalyst favors trapping of the photogenerated electrons from CdS to efficiently react with the preferentially adsorbed water on NbN to effectively produce hydrogen gas.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the NSFC Project (21501052, U1401245, 91622119 and 21371053), the China Postdoctoral Science Foundation (2015M570304), Special Funding for Postdoctoral of Heilongjiang Province (LBH-TZ06019) and the Science Foundation for Excellent Youth of Harbin City of China (2016RQQXJ099) and UNPYSCT-2016173.References
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z. X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731 CAS.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520 RSC.
- K. Maeda and K. Domen, Bull. Chem. Soc. Jpn., 2016, 89, 627 CrossRef CAS.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900 CrossRef CAS PubMed.
- X. Fan, X. Wang, W. Yuan and C. M. Li, Sustainable Energy Fuels, 2017, 1, 2172–2180 CAS.
- S. Xie, Q. Zhang, G. Liu and Y. Wang, Chem. Commun., 2016, 52, 35 RSC.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970 CrossRef CAS PubMed.
- R. Shi, Y. Cao, Y. Bao, Y. Zhao, G. I. N. Waterhouse, Z. Fang, L. Z. Wu, C. H. Tung, Y. Yin and T. Zhang, Adv. Mater., 2017, 29, 1700803 CrossRef PubMed.
- Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Chem. Rev., 2014, 114, 9987 CrossRef CAS PubMed.
- L. Liao, Q. Zhang, Z. Su, Z. Zhao, Y. Wang, Y. Li, X. Lu, D. Wei, G. Feng, Q. Yu, X. Cai, J. Zhao, Z. Ren, H. Fang, F. Robles-Hernandez, S. Baldelli and J. Bao, Nat. Nanotechnol., 2014, 9, 69 CrossRef CAS PubMed.
- X. Wang, L. Yin and G. Liu, Chem. Commun., 2014, 50, 3460 RSC.
- L. Jiang, L. Wang, G. Xu, L. Gu and Y. Yuan, Sustainable Energy Fuels, 2018 10.1039/C7SE00475C.
- S. Bai, J. Jiang, Q. Zhang and Y. Xiong, Chem. Soc. Rev., 2015, 44, 2893 RSC.
- Y. Qu and X. Duan, Chem. Soc. Rev., 2013, 42, 2568 RSC.
- J. Chen, X. J. Wu, L. Yin, B. Li, X. Hong, Z. Fan, B. Chen, C. Xue and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1210 CrossRef CAS PubMed.
- P. R. Jothi, Y. Zhang, J. P. Scheifers, H. Park and B. P. T. Fokwa, Sustainable Energy Fuels, 2017, 1, 1928–1934 CAS.
- J. S. Jang, D. J. Ham, N. Lakshminarasimhan, W. Y. Choi and J. S. Lee, Appl. Catal., A, 2008, 346, 149 CrossRef CAS.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148 RSC.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- S. Chen, S. Shen, G. Liu, Y. Qi, F. Zhang and C. Li, Angew. Chem., Int. Ed., 2015, 54, 3047 CrossRef CAS PubMed.
- A. Korneev, P. Kouminov, V. Matvienko, G. Chulkova, K. Smirnov, B. Voronov and G. N. Gol'tsman, Appl. Phys. Lett., 2004, 84, 5338 CrossRef CAS.
- R. Fujii, Y. Gotoh, M. Y. Liao, H. Tsujia and J. Ishikawa, Vacuum, 2006, 80, 832 CrossRef CAS.
- N. Cansever, M. Danişman and K. Kazmanli, Surf. Coat. Technol., 2008, 202, 5919 CrossRef CAS.
- R. Kaiser, W. Spengle, S. Scricktanz and C. Politis, Phys. Status Solidi B, 1978, 87, 565 CrossRef CAS.
- X. Liu, G. Zhou, S. W. Or, S. Ran and Y. Sun, Ceram. Int., 2015, 41, 849 CrossRef CAS.
- X. Zhang, Y. Lin, D. He, J. Zhang, Z. Fan and T. Xie, Chem. Phys. Lett., 2011, 504, 71 CrossRef CAS.
- Z. Li, P. Luan, X. Zhang, Y. Qu, F. Raziq, J. Wang and L. Jing, Nano Res., 2017, 10, 2321 CrossRef CAS.
- J. Shan, F. Raziq, M. Humayun, W. Zhou, Y. Qu, G. Wang and Y. Li, Appl. Catal., B, 2017, 219, 10 CrossRef CAS.
- Q. Xu, J. Yu, J. Zhang, J. Zhang and G. Liu, Chem. Commun., 2015, 51, 7950 RSC.
- X. L. Wang, W. Liu, Y. Y. Yu, Y. Song, W. Q. Fang, D. Wei, X. Q. Gong, Y. F. Yao and H. G. Yang, Nat. Commun., 2016, 7, 11918 CrossRef CAS PubMed.
- F. Zhang, A. Yamakata, K. Maeda, Y. Moriya, T. Takata, J. Kubota, K. Teshima, S. Oishi and K. Domen, J. Am. Chem. Soc., 2012, 134, 8348 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Raman, XPS, SEM, N2 adsorption–desorption isotherms, DFT calculations and so on. See DOI: 10.1039/c7se00610a |
| This journal is © The Royal Society of Chemistry 2018 |