Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
β-NiS modified CdS nanowires for photocatalytic H2 evolution with exceptionally high efficiency†
Shundong
Guan
ab,
Xiuli
Fu
 *a,
Yu
Zhang
abc and
Zhijian
Peng
*a,
Yu
Zhang
abc and
Zhijian
Peng
 *b
*b
aState Key Laboratory of Information Photonics and Optical Communications, School of Science, Beijing University of Posts and Telecommunications, Beijing 100876, P. R. China. E-mail: xiulifu@bupt.edu.cn; Fax: +86-10-62282054; Tel: +86-10-62282452
bSchool of Science, China University of Geosciences, Beijing 100083, PR China. E-mail: pengzhijian@cugb.edu.cn; Fax: +86-10-82322624; Tel: +86-10-82320255
cSchool of Engineering and Technology, China University of Geosciences, Beijing 100083, PR China
First published on 13th December 2017
Abstract
Co-catalysis is regarded as a promising strategy to improve the hydrogen evolution performance of semiconductor-based photocatalysts. But developing a simple and effective technique to achieve the optimal synergy between co-catalysts and host photocatalysts has been a great challenge. Herein, hybrid photocatalysts consisting of β-NiS modified CdS nanowires (NiS/CdS NWs) have been synthesized via a simple and green hydrothermal route using CdS NWs as the template from thiourea and nickel acetate in the presence of sodium hypophosphite. As a result, a metal Ni intermediate was formed via an electroless plating process assisted by H2PO2−, which facilitated the growth of highly conducting flake-like β-NiS nanostructures onto the surface of the CdS NWs. With the optimal loading amount of NiS, the obtained NiS/CdS NWs present a record-high photocatalytic activity for H2 evolution in lactic acid aqueous solutions under visible light irradiation. At 25 °C, the rate of H2 evolution was measured as 793.6 μmol h−1 (over a 5 mg photocatalyst sample), which is nearly 250-fold higher than that over pure CdS NWs, and the apparent quantum yield reached an exceptionally high value of 74.1% at 420 nm. The mechanism for the photocatalytic H2 evolution over the present NiS/CdS NWs was also proposed. This strategy would provide new insight into the design and development of high-performance heterostructured photocatalysts.
1 Introduction
To alleviate the ever-increasing consumption of fossil fuels and the associated environmental pollution as well as global climate change, considerable efforts have recently been devoted to developing clean, abundant, and renewable energy as an alternative to fossil fuels. Among the various technologies proposed so far, splitting water to produce hydrogen (H2) fuel through sunlight irradiation, during which the solar energy can be converted to storable chemical energy, represents a very promising and sustainable route.1–3 Since Honda and Fujishima reported the photocatalytic splitting of water over TiO2-coated electrodes in 1972,4 extensive investigations have been performed on developing various semiconductor-type photocatalysts. However, it remains a great challenge to obtain highly active, low-cost, and non-toxic photocatalysts capable of catalyzing water splitting under the irradiation of visible light. To improve the photocatalytic H2 evolution reaction (HER) activity, loading a co-catalyst onto the host semiconductor photocatalyst has proven to be an effective strategy, which can dramatically promote the separation of photo-excited charges, and lower the activation energy or overpotential for the reactions.5,6 For example, by introducing a noble metal as the co-catalyst (e.g. Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7,8 or Au
7,8 or Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), the photocatalytic H2 production efficiency becomes much higher than that achieved with bare photocatalysts. Nonetheless, the high cost of noble metals hampers their applications as co-catalysts in practical photocatalytic reactors. Therefore, it is of utmost importance to develop a low-cost, highly efficient noble metal-free photocatalytic HER system.
9), the photocatalytic H2 production efficiency becomes much higher than that achieved with bare photocatalysts. Nonetheless, the high cost of noble metals hampers their applications as co-catalysts in practical photocatalytic reactors. Therefore, it is of utmost importance to develop a low-cost, highly efficient noble metal-free photocatalytic HER system.
In particular, in the last decade, cadmium sulfide (CdS) has received considerable attention for use as an efficient HER photocatalyst due to its suitable direct band-gap (∼2.5 eV) allowing for the absorption of visible light of the solar spectrum, as well as its appropriate conduction band (CB) and valence band (VB) positions, which are thermodynamically favorable for water splitting.10 However, pure CdS does not possess high efficiency for H2 production because of the heavy photo-corrosion under photoelectrochemical conditions.11 Hence, many attempts have been made to modify CdS in order to improve its photocatalytic performance. Specifically, it has been demonstrated that many co-catalysts based on earth-abundant transition metal elements including molybdenum (Mo),12–15 tungsten (W),16–18 cobalt (Co),19–22 nickel (Ni),3,5,23–31 and copper (Cu),32,33 and their corresponding chalcogenides, oxides, and phosphides, could be used in combination with CdS to significantly enhance the photocatalytic efficiency for the HER. Among them, the low band-gap semiconductor, nickel sulfide (NiS), has been considered as a promising alternative to Pt due to its ease of fabrication, low-cost, high power conversion efficiency, high electrical conductivity, and most importantly, friendliness to the environment.34 In the literature, Zhang et al. first prepared a NiS/CdS nanoparticle (NP) hybrid photocatalyst for the HER via a simple hydrothermal loading method, and it showed a high apparent quantum yield (AQY) of 51.3% at 420 nm at room temperature.28 Later on, Qin et al. proposed a one-step hydrothermal approach to synthesize NiS/CdS NPs with an enhanced AQY for the photocatalytic HER up to 60.4% at 420 nm at 35 °C, which was ascribed to the optimized contact between the co-catalyst and host photocatalyst.30
Notwithstanding the remarkable progress, the efficiency of the NiS–CdS composite catalysts reported so far remains unsatisfactory because: (1) CdS and NiS are typical n-type and p-type semiconductors, respectively. It is easy for them to form a p–n junction when they hybridize with each other, which could effectively reduce the recombination rates of photo-generated electrons and holes, thus dramatically enhancing the photocatalytic activity.29 However, in such a p–n junction structure, the photo-generated electrons from CdS cannot be transferred to NiS due to the presence of a built-in electric field from CdS to NiS. As a photocatalyst, CdS in such a composite serves as the active sites of H+ reduction (which is beneficial for hydrogen production), while NiS behaves as the oxidation active sites. In this case, the high electrocatalytic HER activity of NiS commonly observed cannot be expected to contribute to photocatalytic hydrogen production. As a result, the photocatalytic HER performance of such NiS–CdS composite catalysts could only be enhanced to a limited degree;29 (2) although β-NiS has been proven to have higher electrocatalytic activity for hydrogen production than other polymorphs of nickel sulfides due to its higher electrical conductivity, previously reported NiS co-catalyst materials always comprise a mixture of multiple phases with α-NiS and/or nickel polysulfides as the major components, and contain no or very little β-NiS. This is because of the difficulty in synthesizing pure β-NiS via the existing hydrothermal methods;35 (3) the photocatalytic activity is also affected by the morphology of the catalysts. In particular, one-dimensional (1D) nanostructures (e.g. nanowires and nanotubes) offer several advantages, for example, a large surface area resulting from their high aspect ratios, high charge separation and transfer efficiencies, and enhanced light absorption ability, which could substantially improve the activity of the photocatalytic HER.3,36,37 However, the challenge lies in obtaining good contact between the CdS semiconductor host photocatalysts and co-catalysts over the entire 1D structure to enhance the transfer efficiency of photo-generated carriers.
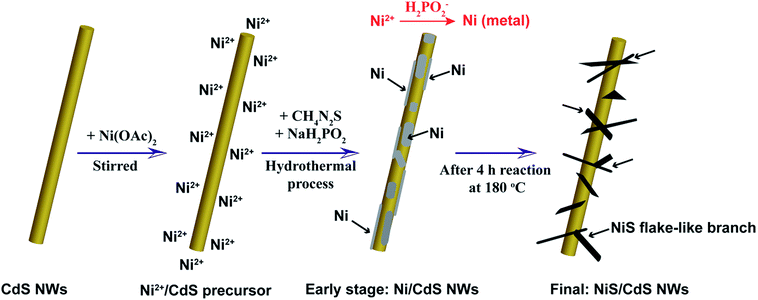
To address these problems, we herein develop a simple and green hydrothermal synthesis route to fabricate a β-NiS modified CdS nanowire (NiS/CdS NW) hybrid photocatalyst using CdS NWs as the scaffold. Our strategy is schematically illustrated in Fig. 1. During the formation of the NiS/CdS hybrid structure, sodium hypophosphite (NaH2PO2) was used as the reducing agent to assist the growth of the NiS co-catalyst through an electrolessly plated Ni film intermediate. As a result, almost pure β-NiS nanoflakes can be obtained, and these are highly dispersed and well adhered onto the surface of the CdS NWs, resulting in a large contact area between the co-catalyst NiS nanoflakes and host CdS NWs. Due to the high electrical conductivity of β-NiS, fast charge separation between photo-generated electron–hole pairs and high carrier transfer efficiency were realized, which were able to greatly enhance the photoelectric conversion efficiency of the composite catalyst. Besides, such an electroless plating process could effectively retain the formation of a p–n junction between CdS and β-NiS in this hybrid structure. In this case, the highly electrocatalytically active β-NiS would also serve as the active sites for photocatalytic H2 production, because the photo-generated electrons from the CdS NWs could be easily transferred to β-NiS, namely, the reduction of H+ to H2 can also proceed on the surface of β-NiS, thus maximizing the photocatalytic HER activity of the composite catalyst. By optimizing the loading of NiS, the NiS/CdS NW hybrid catalysts can achieve a record-high photocatalytic activity among all the CdS-based noble metal-free photocatalysts for H2 evolution under visible light irradiation (λ ≥ 420 nm). The rate of H2 evolution was measured to be 592.1 μmol h−1 (over a 5 mg photocatalyst sample) at 7 °C, and the AQY was 57.8% at 420 nm. When the reaction temperature was maintained at 25 °C, the yield and AQY were further increased up to 793.6 μmol h−1 and 74.1%, respectively. Such high photocatalytic activity can be attributed surely to the optimized synergistic effect between the highly reactive β-NiS and CdS NWs. In addition, this simple, cost-effective, and environmentally friendly strategy would provide new insight into the design and development of high-performance heterostructured photocatalysts.
2 Experimental
2.1 Chemicals and materials
Cadmium acetate dihydrate (Cd(CH3COO)2·2H2O, 98.0+%), sulfur powder (S, 99.5%), nickel acetate tetrahydrate (Ni(CH3COO)2·4H2O, 98.0+%), sodium hypophosphite monohydrate (NaH2PO2·H2O, 98.0–103.0%), lactic acid (C3H6O3, 85.0+%), and sodium sulfite anhydrous (Na2SO3, 97.0+%) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Thiourea (CH4N2S, 99.0+%) and sodium sulfide nonahydrate (Na2S·9H2O, 98.0+%) were bought from Xilong Scientific Co., Ltd (Shantou, China). Ethylenediamine (C2H8N2, 99.0+%) was purchased from Tianjin Fuchen Chemical Reagents (Tianjin, China). All chemicals were used as received without further purification.2.2 Preparation of CdS NWs
CdS NWs were fabricated according to a previous report with some modifications.38 Typically, 0.2665 g of Cd(CH3COO)2·2H2O and 0.0641 g of sulfur were dispersed in 40 mL of ethylenediamine under vigorous stirring and then transferred into a 50 mL Teflon-lined autoclave. The autoclave was heated up to 200 °C, maintained at this temperature for 20 h, and then cooled down naturally to room temperature. Finally, the resultant precipitate (CdS NWs) was washed and then dispersed in deionized water for use.2.3 Preparation of NiS/CdS NWs and NiS nanostructures
To synthesize the proposed NiS/CdS NW hybrid photocatalyst, 29 mg (∼0.2 mmol) of CdS NWs (aqueous suspension, where the concentration was estimated on the basis of dry powders) and a designated amount of Ni(CH3COO)2·4H2O were dispersed in 50 mL of deionized water, and stirred for 3 h. Then, an appropriate amount of thiourea in a Ni/S feed molar ratio (FMR) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 0.6 mmol of NaH2PO2·H2O were added into the solution and kept stirring vigorously. Subsequently, the mixture was transferred to a 100 mL Teflon-lined autoclave and solvothermally treated at 180 °C for 4 h, for which it was heated from room temperature to 180 °C in ca. 40 min. After the autoclave was cooled down naturally to room temperature, the precipitate was collected and washed with distilled water and ethanol respectively two times. Finally, the product was dispersed in ethanol for use. In this work, the NiS loading was adjusted by changing the Ni/Cd FMR in the range of 0.2 to 1.2 for the reactions, while all the other conditions were kept unchanged.
4 and 0.6 mmol of NaH2PO2·H2O were added into the solution and kept stirring vigorously. Subsequently, the mixture was transferred to a 100 mL Teflon-lined autoclave and solvothermally treated at 180 °C for 4 h, for which it was heated from room temperature to 180 °C in ca. 40 min. After the autoclave was cooled down naturally to room temperature, the precipitate was collected and washed with distilled water and ethanol respectively two times. Finally, the product was dispersed in ethanol for use. In this work, the NiS loading was adjusted by changing the Ni/Cd FMR in the range of 0.2 to 1.2 for the reactions, while all the other conditions were kept unchanged.
As for the pure NiS nanostructures, they were synthesized by a similar hydrothermal reaction without the presence of CdS NWs.
2.4 Materials characterization
The phase composition of the obtained products was identified via grazing incidence X-ray diffraction (GI-XRD, D/max-RB, Japan; Cu Kα radiation, λ = 1.5418 Å) in a continuous scanning mode with a scanning rate of 6° min−1 and an X-ray incidence angle of 1°. The morphology and structure were examined by field emission scanning electron microscopy (FE-SEM, S4800, Hitachi, Japan), transmission electron microscopy (TEM, Tecnai G2 F20 U-TWIN, FEI, America) and high-resolution TEM (HRTEM). The chemical composition was measured by an energy dispersive X-ray (EDX) spectrometer attached to the TEM instrument. The chemical state of the elements in the samples was investigated by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB MKII, Thermo VG Scientific Ltd., UK), and the results were calibrated by the C 1s line (binding energy, 284.8 eV). The UV-visible absorption spectra were recorded on a Varian Cary 5000 UV-vis spectrophotometer (Agilent, America).2.5 Photocatalytic H2 evolution
Photocatalytic water splitting was carried out in a LabSolar photocatalytic H2 evolution system (Perfectlight, Beijing, China) equipped with a 300 W Xe lamp (MICROSOLAR300, Perfectlight, Beijing, China). In a typical photocatalytic reaction, 5 mg of the catalysts was dispersed in an aqueous solution (100 mL) containing 20 vol% lactic acid. After that, the system was sealed and pumped out to a vacuum level of −0.1 MPa. During the reaction, the circulating cooling water system worked so as to keep the reaction at 7 or 25 °C, and the reactor, under magnetic stirring, was irradiated by visible light (λ ≥ 420 nm) provided by the 300 W Xe lamp with an UV cut-off filter. The distance between the Xe lamp and the surface of the reaction solution was kept at 20 cm, and the effective irradiation area was measured as 12.57 cm2. The gases evolved were analyzed on-line with a gas chromatograph with N2 as the carrier gas (GC-7900, Xuansheng Scientific Instrument Co. Ltd, Shanghai, China).The AQY was calculated according to eqn (1) using a 300 W Xe lamp with a band-pass filter (λ = 420 ± 5 nm) and an irradiatometer (FZ-A, Photoelectric Instrument Factory of Beijing Normal University, Beijing, China):
 | (1) |
The measured power of the light was 5.52 mW cm−2 and the irradiation area was 12.57 cm2. So, the corresponding number of incident photons was 1.466 × 1017 photons per second.
2.6 Photoelectrochemical measurements
3 Results and discussion
3.1 Photocatalytic performance for H2 evolution
The photocatalytic performance for H2 evolution of the NiS/CdS NWs prepared at different Ni/Cd FMRs was first evaluated under visible light irradiation at 7 °C, and the result is shown in Fig. 2a. For comparison, the performance of pure CdS NWs (i.e. NWs prepared at a Ni/Cd FMR of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and NiS nanostructures (i.e. those prepared at a Ni/Cd FMR of 1
1) and NiS nanostructures (i.e. those prepared at a Ni/Cd FMR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
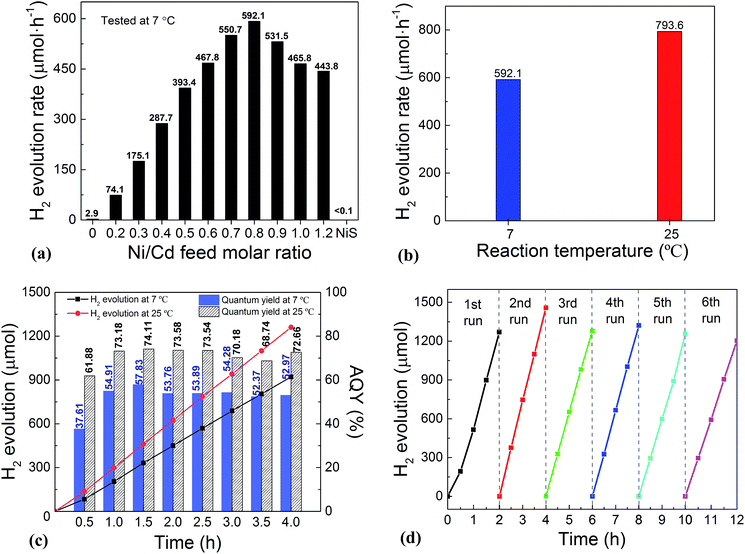
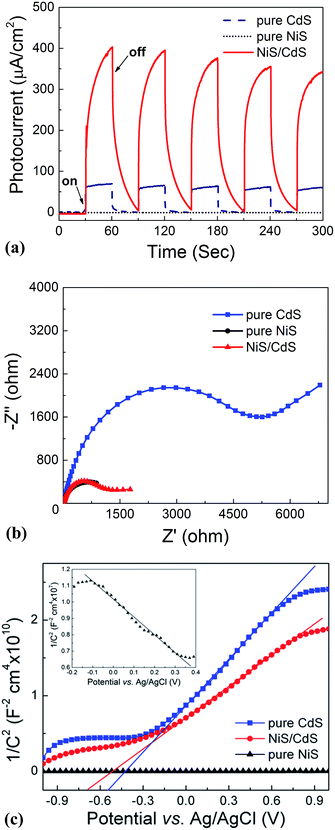
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 via a similar hydrothermal process without a CdS template) was also investigated. For the HER, after a series of optimizations, 20 vol% lactic acid was chosen as the sacrificial agent (Fig. S1 and S2, ESI†). As is seen from Fig. 2a, the photocatalytic HER rate of the pure CdS NWs was rather low (2.9 μmol h−1), presenting a poor photocatalytic activity as reported in the literature.3,28 When NiS was loaded onto the CdS NWs, the photocatalytic HER rate was significantly increased. And increasing the loading amount of NiS onto CdS through increasing the Ni/Cd FMR (see the practical molar percentage of NiS for each sample in Table S1†) first led to an increase and then a decrease in the H2 evolution rate, with the highest HER rate of 592.1 μmol h−1 achieved by the NiS/CdS hybrid photocatalyst prepared at a Ni/Cd FMR of 0.8, which is ca. 204-fold higher than that of pure CdS NWs. As for the hybrid photocatalysts with a higher content of NiS (prepared at a Ni/Cd FMR higher than 0.8), the H2 evolution rate decreased gradually as the loading of NiS increased, which might be due to the shielding effect of excessive NiS.29,30 Consequently, in the subsequent experiments, our focus was placed on the optimized photocatalysts prepared at a Ni/Cd FMR of 0.8. In order to examine whether the enhancement in HER activity observed for the NiS/CdS hybrid photocatalyst results merely from NiS, the photocatalytic HER of pure NiS nanostructures was also investigated. As is seen from Fig. 2a, there was no appreciable amount of H2 detected when the pure NiS nanostructures were used, revealing that NiS alone was not an active photocatalyst for H2 evolution, but served only as a co-catalyst here. Therefore, the enhanced HER activity should be attributed to the synergistic effect between NiS and CdS NWs.
0 via a similar hydrothermal process without a CdS template) was also investigated. For the HER, after a series of optimizations, 20 vol% lactic acid was chosen as the sacrificial agent (Fig. S1 and S2, ESI†). As is seen from Fig. 2a, the photocatalytic HER rate of the pure CdS NWs was rather low (2.9 μmol h−1), presenting a poor photocatalytic activity as reported in the literature.3,28 When NiS was loaded onto the CdS NWs, the photocatalytic HER rate was significantly increased. And increasing the loading amount of NiS onto CdS through increasing the Ni/Cd FMR (see the practical molar percentage of NiS for each sample in Table S1†) first led to an increase and then a decrease in the H2 evolution rate, with the highest HER rate of 592.1 μmol h−1 achieved by the NiS/CdS hybrid photocatalyst prepared at a Ni/Cd FMR of 0.8, which is ca. 204-fold higher than that of pure CdS NWs. As for the hybrid photocatalysts with a higher content of NiS (prepared at a Ni/Cd FMR higher than 0.8), the H2 evolution rate decreased gradually as the loading of NiS increased, which might be due to the shielding effect of excessive NiS.29,30 Consequently, in the subsequent experiments, our focus was placed on the optimized photocatalysts prepared at a Ni/Cd FMR of 0.8. In order to examine whether the enhancement in HER activity observed for the NiS/CdS hybrid photocatalyst results merely from NiS, the photocatalytic HER of pure NiS nanostructures was also investigated. As is seen from Fig. 2a, there was no appreciable amount of H2 detected when the pure NiS nanostructures were used, revealing that NiS alone was not an active photocatalyst for H2 evolution, but served only as a co-catalyst here. Therefore, the enhanced HER activity should be attributed to the synergistic effect between NiS and CdS NWs.
In consideration of the stability of the whole photocatalytic HER and the protection of the gas chromatograph from vapor erosion, most of our tests were conducted at 7 °C stabilized by a circulating cooling water system. But for comparison with literature reports, the photocatalytic HER activity of the optimized NiS/CdS NWs was also investigated at 25 °C. As is shown in Fig. 2b, when the reaction temperature was increased to 25 °C, the photocatalytic HER rate was enhanced up to 793.6 μmol h−1, which could be attributed to the easier desorption of both the generated H2 and the oxidized lactic acid molecules from the surface of the photocatalyst at a higher temperature.39
To further investigate the AQY for the photocatalytic HER, 5 mg of the optimized NiS/CdS NWs was dispersed in 100 mL of aqueous solution containing 20 vol% lactic acid, and then the solution was irradiated by visible light (λ = 420 ± 5 nm) provided by a 300 W Xe lamp with a band-pass filter at 7 and 25 °C. As is seen from Fig. 2c, the amount of the generated H2 was increased gradually over time, but the AQYs did not vary substantially after 1 h of irradiation, and these were roughly 55% at 7 °C and 73% at 25 °C. However, those values were lower in the first half hour (about 37.61% at 7 °C and 61.88% at 25 °C, respectively), which might be due to an induction period at the early reaction stage and the dissolution of H2 in the solution.3 The highest AQYs were obtained at 1.5 h at both 7 and 25 °C, reaching 57.83% and 74.11%, respectively, which, to the best of our knowledge, are the highest values among all the CdS-based noble metal-free photocatalysts (Table S2†). From these results, it can be concluded that the loading of NiS can significantly improve the photocatalytic H2 evolution performance of CdS NWs with a much higher HER rate and more efficient conversion of visible light energy.
The stability and reusability of the obtained NiS/CdS NW hybrid photocatalysts were evaluated through six consecutive runs of the HER under the same conditions. Each cycle was performed under visible light irradiation (λ ≥ 420 nm) for 2 h. After each run, the reaction system was re-evacuated. Fig. 2d displays the recycling performance of the photocatalytic HER over the optimized NiS/CdS NWs. The result reveals that there was no significant decrease in the H2 evolution ability after each cycle. The optimized NiS/CdS NW hybrid photocatalyst could maintain a similar photocatalytic activity for more than 12 h, indicating an excellent stability of the present hybrid for the photocatalytic HER.
3.2 Composition, structure, and synthesis mechanism
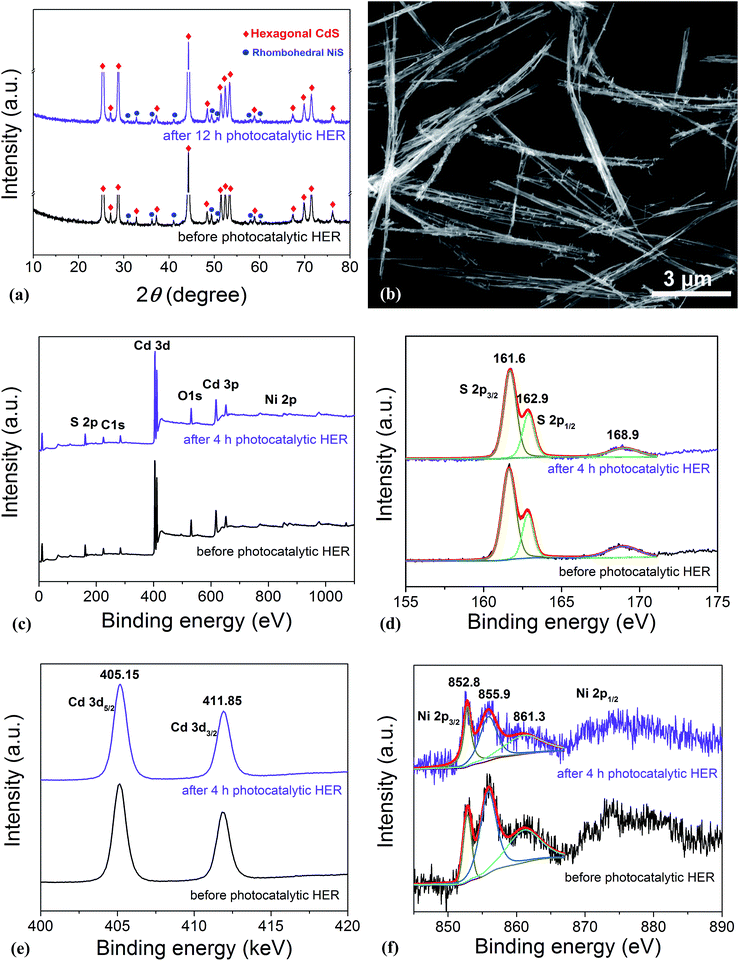
In order to disclose the origin of the high photocatalytic HER efficiency observed for the hybrid NiS/CdS NWs, their compositions and structures were comprehensively investigated. Fig. 3 displays the XRD pattern of the optimized NiS/CdS NW hybrid photocatalyst, in comparison to that of the pure CdS NWs (Fig. S3†) and pure NiS nanostructures (Fig. S4†). For the pure CdS sample, the XRD pattern presents sharp diffraction peaks, indicating its good crystallinity. And all the diffraction peaks of this sample could be indexed to those of the hexagonal CdS phase (JCPDS no. 77-2306). For the pure NiS nanostructures, the main diffraction peaks matched well with those of the rhombohedral NiS phase (β-NiS, JCPDS no. 86-2281), while there were also some weak peaks (marked by green squares) with 2θ values of 34.67°, 45.91°, and 53.55° that can be assigned to the (101), (102), and (110) crystal planes, respectively, of the hexagonal NiS phase (α-NiS, JCPDS no. 75-0613). These results indicate that the pure NiS sample consists of β-NiS as the major phase and α-NiS as the minor phase. After NiS was loaded onto the CdS NWs, the major sharp diffraction peaks in the XRD pattern could be attributed to the CdS NWs (hexagonal, corresponding to JCPDS no. 77-2306), while several relatively weak peaks (marked by blue dots) could be observed at 2θ values of 30.31°, 32.21°, 35.71°, 40.47°, 48.84°, 50.14°, 57.43°, and 59.70°, matching well with the (101), (300), (021), (211), (131), (410), (330), and (012) crystal planes of β-NiS, respectively. In this case, no obvious peaks from α-NiS could be detected. This result is different from those reported previously in the literature about NiS/CdS photocatalysts, where the loaded NiS co-catalysts consisted of mainly α-NiS and a little of other phases such as β-NiS and nickel polysulfides (e.g. Ni3S4).29,30 As is known, compared to α-NiS, β-NiS has a smaller charge transfer resistance, which is beneficial for electronic transport through the material system, suggesting better activity for the HER.35 Thus, the presence of the almost pure β-NiS phase in the present NiS/CdS NW hybrid photocatalyst may be one of the most important reasons for its high visible light-driven HER activity.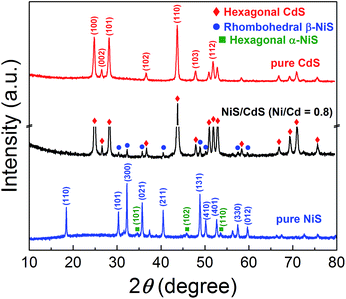 | ||
| Fig. 3 XRD patterns of the obtained pure CdS NWs, pure NiS nanostructures, and optimized NiS/CdS NWs. | ||
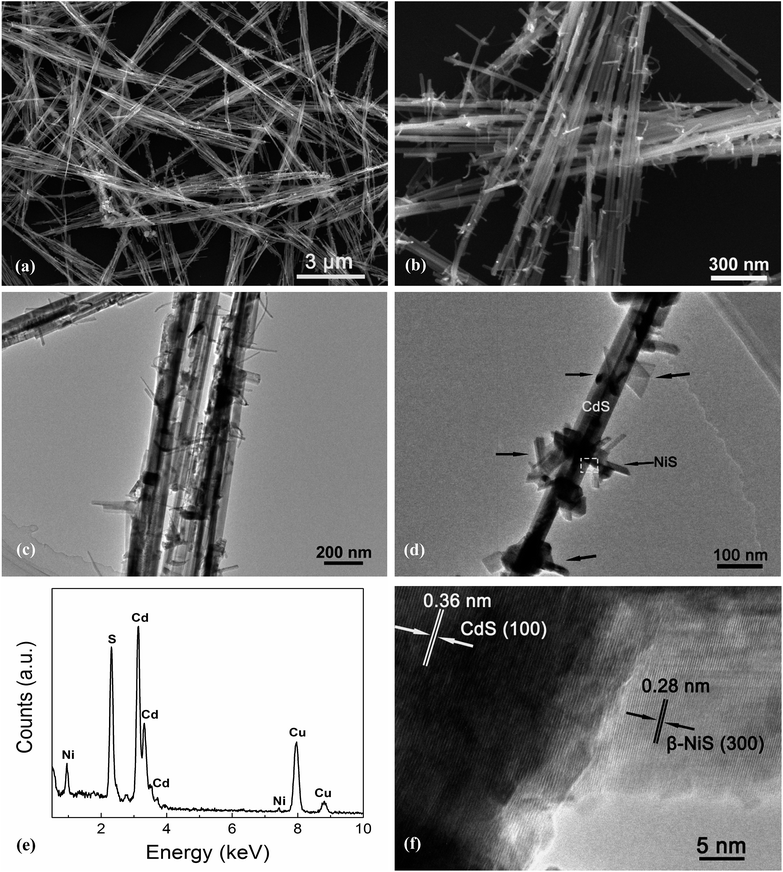
Fig. 4 exhibits the results of the microstructural characterization of the optimized NiS/CdS NWs. The SEM images shown in Fig. 4a and b reveal that the optimized NiS/CdS NWs have an average diameter of about 30 nm and a length of 5–10 μm. Such a high aspect ratio was inherited from the parent CdS NWs (Fig. S3†), implying that the synthesized NiS/CdS hybrid NWs would also have a large specific surface area as with the pure CdS NWs.3,37 Furthermore, there were no bulky clusters around the NWs; instead, some small flake-like branches could be observed on the surface of the obtained NWs, resulting in a larger contact area between NiS and the CdS NWs. By comparison with the SEM images of the pure NiS nanostructures (Fig. S4†), in which many flower-like nanosheets as well as some NPs could be observed, it can be deduced that the small flake-like branches on the surface of the obtained NWs might be β-NiS.
Further TEM observation (Fig. 4c and d) shows a consistent average diameter and length for the obtained NiS/CdS composite, and good dispersity of the flake-like branches on the surface of the CdS NWs. Besides, the EDX spectrum (Fig. 4e) of the optimized NiS/CdS NWs reveals the presence of Ni, S, Cd, and Cu, implying that the NiS co-catalyst was successfully loaded on the CdS NWs. As for Cu, the signal in the spectrum originated from the copper grid to support the TEM sample. More EDX examinations were also conducted at a single branch and the optimized NiS/CdS NWs, respectively, and the results are shown in Fig. S5.† These results confirm that the flake-like branches are indeed NiS, and the branches are well adhered to and densely dispersed onto the surface of the CdS NWs. To obtain more details about the contact area between the NiS flakes and CdS NWs, a HRTEM image corresponding to the square area shown in Fig. 4d is presented in Fig. 4f. Two distinct sets of lattice fringes can be identified from this image. The inter-planar spacing of 0.36 nm can be assigned to the (100) lattice plane of hexagonal CdS (JCPDS no. 77-2306), and that of 0.28 nm is attributed to the (300) plane of β-NiS (JCPDS no. 86-2281), matching well with the XRD results as discussed above, and indicating a good adhesion between the NiS flakes and CdS NWs. All these SEM and TEM results revealed that the highly reactive β-NiS co-catalyst was successfully loaded on the surface of the CdS NWs with a dense dispersion and intimate contact, which is beneficial for enhancing the HER efficiency of the synthesized hybrid photocatalysts.
To investigate why highly reactive β-NiS flakes, instead of other polymorphs, were formed, and why the β-NiS flakes were highly dispersed on and well adhered to the surface of the CdS NWs, several control experiments were conducted, with the aim of loading NiS onto the CdS NWs without the presence of NaH2PO2·H2O while keeping the other synthesis conditions fixed. The recorded XRD pattern (Fig. S6a†) shows that no NiS could be identified in the sample when the Ni/S FMR was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and the Ni/Cd FMR was at 0.8. Unexpectedly, a new weak peak could be identified at a 2θ value of 19.21°, which was indexed to hexagonal Ni(OH)2 (JCPDS no. 73-1520), indicating that a small amount of thiourea could not provide enough S to form NiS due to the slow decomposition rate of thiourea.28 And the formation of Ni(OH)2 might be attributed to the hydrolysis of Ni(CH3COO)2 under hydrothermal conditions, as demonstrated in ref. 40. However, when excessive thiourea (with a Ni/S FMR of 1
4 and the Ni/Cd FMR was at 0.8. Unexpectedly, a new weak peak could be identified at a 2θ value of 19.21°, which was indexed to hexagonal Ni(OH)2 (JCPDS no. 73-1520), indicating that a small amount of thiourea could not provide enough S to form NiS due to the slow decomposition rate of thiourea.28 And the formation of Ni(OH)2 might be attributed to the hydrolysis of Ni(CH3COO)2 under hydrothermal conditions, as demonstrated in ref. 40. However, when excessive thiourea (with a Ni/S FMR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, similar to the experiments in ref. 28–31) was used in the reaction but still without adding NaH2PO2·H2O, NiS2 and Ni3S4 were formed instead of NiS (Fig. S6b†). Such products were separately formed or aggregated together rather than being dispersed homogeneously onto the surface of the CdS NWs (Fig. S6c†), which would lead to poor contact between the co-catalysts and host photocatalysts. The photocatalytic activities of the samples prepared without NaH2PO2·H2O were also investigated (Fig. S6d†), both of which show activities lower than the one fabricated in the presence of NaH2PO2·H2O. According to these control experiments, it can be deduced that the applied NaH2PO2·H2O played a critical role in the synthesis of the hybrid NiS/CdS NWs.
20, similar to the experiments in ref. 28–31) was used in the reaction but still without adding NaH2PO2·H2O, NiS2 and Ni3S4 were formed instead of NiS (Fig. S6b†). Such products were separately formed or aggregated together rather than being dispersed homogeneously onto the surface of the CdS NWs (Fig. S6c†), which would lead to poor contact between the co-catalysts and host photocatalysts. The photocatalytic activities of the samples prepared without NaH2PO2·H2O were also investigated (Fig. S6d†), both of which show activities lower than the one fabricated in the presence of NaH2PO2·H2O. According to these control experiments, it can be deduced that the applied NaH2PO2·H2O played a critical role in the synthesis of the hybrid NiS/CdS NWs.
Based on all the aforementioned discussions, the formation mechanism of the present NiS/CdS NW hybrid structure is proposed as follows (also see Fig. 1). When Ni(CH3COO)2 was added into the CdS NW suspension under magnetic stirring, the hydrolyzed Ni2+ could be adsorbed onto the CdS NWs to form a Ni2+/CdS precursor due to the presence of a lot of cation vacancies on the surface of the CdS NWs.41 At the early stage of the subsequent hydrothermal process, although the thiourea was not decomposed yet at low temperature, a small quantity of Ni2+ or Ni(CH3COO)2 had been already reduced into metallic Ni by H2PO2−via a process as follows:42
| Ni2+ + 2H2PO2− + 2H2O → Ni + 2H2PO3− + 2H+ + H2 | (2) |
| Ni(CH3COO)2 + 2H2PO2− + 2H2O → Ni + 2H2PO3− + 2H+ + H2 + 2CH3COO−, | (3) |
In addition, in ref. 35, it had been proven that decreasing the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio in the precursor would facilitate the formation of highly reactive β-NiS. However, a small amount of thiourea cannot provide enough S to form NiS due to the slow decomposition rate of thiourea, as discussed above. When NaH2PO2·H2O is added in, H2PO2− can accelerate the decomposition of thiourea,42 which would facilitate the formation of β-NiS even with a low concentration of thiourea under the present synthesis conditions. Consequently, almost pure β-NiS was formed and it was highly dispersed on and well adhered to the surface of the CdS NWs.
S ratio in the precursor would facilitate the formation of highly reactive β-NiS. However, a small amount of thiourea cannot provide enough S to form NiS due to the slow decomposition rate of thiourea, as discussed above. When NaH2PO2·H2O is added in, H2PO2− can accelerate the decomposition of thiourea,42 which would facilitate the formation of β-NiS even with a low concentration of thiourea under the present synthesis conditions. Consequently, almost pure β-NiS was formed and it was highly dispersed on and well adhered to the surface of the CdS NWs.
As is well known, stability and repeatability are very important aspects of a photocatalyst. Thus, the phase composition and morphology of the photocatalysts after a recycling photocatalytic HER were investigated, and the results are shown in Fig. 5a and b, respectively. These results reveal that there is no obvious change in both the morphology and phase composition of the photocatalyst after a 12 h consecutive reaction, which may be due to the good adhesion between NiS and CdS and the high crystallinity of the β-NiS. Besides, the chemical bonding states of the elements in the photocatalysts before and after the HER were also examined by XPS. As shown in Fig. 5c, the XPS survey spectra indicate the presence of Cd, S, Ni, O, and C in both samples. The carbon peak is attributed to the hydrocarbon in the XPS instrument itself. The two peaks for S 2p3/2 in the narrow S spectrum (Fig. 5d) at 161.6 and 162.9 eV are close to the binding energy of S2− in CdS and NiS, respectively.28 The weak peak at 168.9 eV may be assigned to the small amount of S in a higher valence state, which came from the hydrolysis of thiourea. And the two peaks of Cd 3d (Fig. 5e) at 405.15 and 411.85 eV are attributed to the Cd 3d5/2 and Cd 3d3/2 in CdS, respectively.23 The high-resolution Ni 2p spectra are shown in Fig. 5f. The peak at 852.8 eV is close to the reported value for NiS,43 and the two peaks at 855.9 and 861.3 eV can be assigned to the main and satellite peaks of Ni2+ in Ni(OH)2,27 which came from the hydrolysis of Ni(CH3COO)2 under hydrothermal conditions, as discussed above. But Ni(OH)2 was not detected by XRD, SEM, and TEM characterizations in the samples, which might be due to the fact that Ni(OH)2 was of poor crystallinity and/or it was absorbed on the surface of the samples with an amorphous structure. Moreover, as compared in Fig. S6d,† the Ni(OH)2 modified CdS displays much lower photocatalytic HER activity than the optimized NiS/CdS sample, revealing that the high activity of the present hybrid photocatalysts is mainly derived from NiS instead of Ni(OH)2. In addition, after the 4 h photocatalytic HER, the peaks at 855.9 and 861.3 eV have markedly attenuated, indicating the decreased content of Ni(OH)2, which might result from its decomposition with acidic corrosion in lactic acid. However, there is no obvious attenuation in the peak at 852.8 eV, suggesting the good chemical stability of NiS during the photocatalytic HER. In addition, from Fig. 5d–f, no obvious changes in the binding energy of the Cd, S, and Ni elements can be observed before and after the 4 h photocatalytic HER, revealing that there is no valence change in these elements and implying that the chemical stability of the present photocatalyst is very high. In summary, the excellent stability in the morphology, composition, and surface chemistry contributes significantly to the outstanding photocatalytic HER activity of the present NiS/CdS NW hybrid photocatalyst.
3.3 Optoelectrochemical properties and photocatalytic HER mechanism
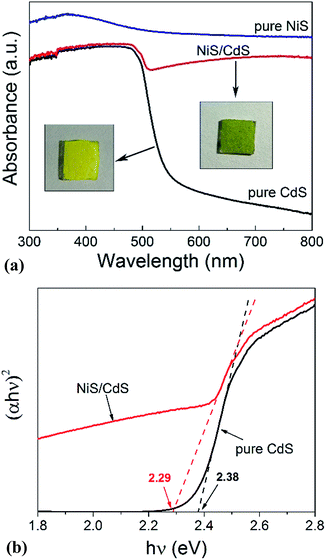
In order to shed more light on the mechanism of the photocatalytic HER over the present NiS/CdS NW hybrid photocatalyst, the optoelectrochemical properties were further examined in comparison to those of the obtained pure CdS NWs and NiS nanostructures. Fig. 6a displays the UV-visible absorption spectra of pure CdS NWs, pure NiS nanostructures, and the optimized NiS/CdS NWs. As seen from this figure, the onset of the absorption edge of the pure CdS NWs is located at about 520 nm, which is in good agreement with the reported value in the literature.28–30 However, after β-NiS was loaded onto the CdS NWs, the absorption in the visible light region after 510 nm was substantially enhanced, which can be confirmed by the colour change of both samples (insets in Fig. 6a). These results indicate that the loading of the β-NiS co-catalyst can effectively broaden the region of light absorption. The enhanced light absorption can be attributed to the presence of the low band-gap black NiS in the NiS/CdS NW composite, which has a strong broad absorption in the range of 300–800 nm (Fig. 6a). In addition, a slight red-shift of the absorption edge compared to that of the pure CdS NWs could be observed, indicating that there is a decrease in the band-gap energy (Eg) of the optimized NiS/CdS hybrid photocatalyst. Furthermore, the band-gaps of both samples were estimated from their Tauc plots as shown in Fig. 6b, i.e. the curves of (αhν)rversus photon energy (hν) derived from the UV-vis spectra, in which r = 2 because CdS is a direct band-gap semiconductor, by measuring the x-axis intercept of the extrapolated line from the linear region of the curve. The calculated Eg values of the pure CdS NWs and optimized NiS/CdS NWs were 2.38 and 2.29 eV, respectively. As expected, a decrease in Eg was determined, indicating that some Ni2+ ions might be doped into the CdS lattice. For the doping, the cation exchange reaction, Ni2+ + CdS → Cd2+ + NiS, is not a favorable route, because NiS has a much larger solubility than CdS.28 Instead, doping may happen through the formation of metallic Ni during the hydrothermal reaction as in the above-mentioned synthesis mechanism, which will give rise to the reaction of Ni0 + CdS → Cd0 + NiS.44Moreover, the investigation on the transient photocurrent as shown in Fig. 7a reveals that, compared to the optimized NiS/CdS NWs, pure CdS NWs exhibit a weaker photocurrent, which could be attributed to the easy recombination of photo-generated electron–hole pairs in pure CdS. But when β-NiS was loaded onto the surface of the CdS NWs, the photocurrent of the samples was significantly enhanced. On the other hand, no obvious photocurrent was detected on the electrode coated by pure NiS nanostructures. These results reveal that the loaded β-NiS onto the CdS NWs has no significant contribution to the generation of photocarriers, in spite of its strong broad absorption in the range of 300–800 nm (Fig. 6a). Moreover, this conclusion is also supported by the IPCE measurements. As is seen in Fig. S7,† under light irradiation in the range of 400–520 nm, pure β-NiS nanostructures do not show any photo-to-electron conversion, while pure CdS NWs only present a low photon-to-electron conversion efficiency. However, after the loading of β-NiS onto the CdS NWs, the photon-to-electron conversion efficiency is dramatically enhanced. Meanwhile, under light irradiation above 520 nm, all three samples (pure CdS, pure NiS, and the NiS/CdS hybrid structure) have a very low photon-to-electron conversion efficiency. The aforementioned facts corroborate that in the present NiS/CdS NW hybrids, β-NiS is not a photocatalyst but only serves as a co-catalyst for CdS NWs. Such a co-catalyst would promote the separation of photo-generated electron–hole pairs and enhance the transfer efficiency of photo-generated carriers.
In addition, the EIS Nyquist plots (Fig. 7b) reveal that the impedance of pure CdS NWs is the highest, compared to the quite smaller values for the pure NiS nanostructures and the optimized NiS/CdS NWs, confirming the high charge transfer rate in the hybrid NiS/CdS NW sample after loading β-NiS onto the CdS NWs. This phenomenon should stem from the intimate contact between the loaded β-NiS and CdS NWs, and the high conductivity of β-NiS. All these results indicate that loading β-NiS onto CdS NWs is beneficial for the photocatalytic HER.
To further investigate the transfer of photo-generated charge carriers in the NiS/CdS NW hybrid structure, impedance spectroscopy was performed at a fixed AC frequency of 1 kHz to acquire the M–S plot. As shown in Fig. 7c, the pure CdS NWs present a positive slope, while the pure NiS nanostructures have a negative one (inset in Fig. 7c), indicating an n-type and p-type semiconductor behaviour for the two samples, respectively. But for the NiS/CdS NW hybrid structure, the M–S plot also shows a positive slope in the whole range without any p–n junction characteristic (e.g., with a “V-shape” M–S plot), indicating that there is no p–n junction formed between the n-type CdS NWs and p-type β-NiS. As a result, the photo-generated electrons can be easily transferred from CdS to NiS. This is completely different from the previously reported NiS/CdS composites in the literature.29,45 And this phenomenon should be attributed to the electroless plating process during the formation of the NiS/CdS NW hybrid structure, as discussed above.
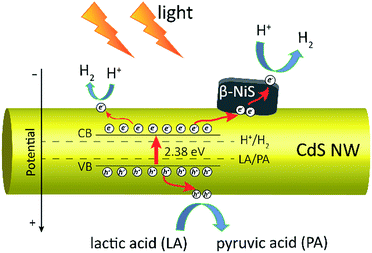
Based on all the above observations and discussions, the photocatalytic HER mechanism over the hybrid NiS/CdS NWs can be depicted as in Fig. 8. As can be seen, under visible light irradiation, the photo-generated electrons (e−) jump into the CB of CdS, leaving holes (h+) in the VB. The photo-generated electrons can partially move toward the surface of CdS, directly reducing H+ in the solution from water or lactic acid to H2. Because no p–n junction exists in the present NiS/CdS hybrid structure, the photo-generated electrons can be easily transferred to the surface of β-NiS due to the intimate contact between the host CdS and co-catalyst β-NiS and high electrical conductivity of β-NiS. As a result, the separation of photo-generated electron–hole pairs is substantially improved. Subsequently, the electrons on the surface of β-NiS can reduce the H+ in the solution to H2 effectively due to the high electrocatalytic HER activity of β-NiS. This process can be illustrated in eqn (4)–(6):28
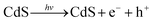 | (4) |
| NiS + H+ + e− → HNiS | (5) |
| HNiS + H+ + e− → NiS + H2 | (6) |
In this case, the highly electrocatalytically active β-NiS would also serve as the active sites for the photocatalytic HER. In other words, the reduction of H+ to H2 can also proceed on the surface of β-NiS, thus maximizing the photocatalytic HER activity of the present NiS–CdS composite catalyst. At the same time, the photo-generated h+ can be absorbed effectively by lactic acid on the surface of CdS, which can oxidize the lactic acid to pyruvic acid. And such a process has been proven to be the only pathway for the consumption of photo-generated holes.28 Moreover, the presence of the β-NiS co-catalyst also enhanced the visible light-harvesting ability of the NiS/CdS hybrid catalyst (Fig. 6a), which might be another reason for the enhanced photocatalytic activity. In summary, the dramatically enhanced photocatalytic HER activity of the NiS/CdS hybrid catalyst can be attributed to the synergistic effect of the enhanced visible light-harvesting ability and highly effective separation of photo-generated electron–hole pairs, resulting from the loading of the β-NiS co-catalyst.
In addition, for many photocatalysts based on CdS,3,21,23 Na2SO3 and Na2S could be used as the sacrificial agents, presenting high HER activities. However, for the photocatalytic system over the present NiS/CdS NW hybrid catalyst, the HER activities in Na2SO3 and Na2S solutions were much lower than that in lactic acid aqueous solution (Fig. S1†). It seems that the acidic conditions may facilitate the photocatalytic HER over the hybrid NiS/CdS NWs, possibly because the high concentration of H+ in the acidic solution could promote the reactions of eqn (5) and (6).
4 Conclusions
A β-NiS modified CdS NW hybrid structure was synthesized via a novel hydrothermal synthesis method. The as-obtained NiS/CdS NWs show high photocatalytic HER activity under visible light irradiation. The HER rate measured at 7 °C over the optimal NiS/CdS NWs prepared at a Ni/Cd feed molar ratio of 0.8 was 592.1 μmol h−1, and the AQY at 420 nm was 57.8%. When the reaction temperature was increased to 25 °C, they could be further improved up to 793.6 μmol h−1 and 74.1%, respectively. To the best of our knowledge, the present system exhibits the highest AQY among all the CdS-based noble metal-free photocatalysts.In the present synthesis route, NaH2PO2·H2O plays a critical role in achieving such highly active NiS/CdS NW hybrid photocatalysts. It is proposed that a metallic Ni film intermediate is first formed via an electroless plating process assisted by H2PO2−, which would then induce the growth of β-NiS flake-like branches on the surface of the CdS NWs with high dispersion and intimate contact. Meanwhile, H2PO2− could also accelerate the decomposition of thiourea, further facilitating the formation of highly active β-NiS at a low concentration of thiourea.
The mechanism for the photocatalytic HER over the present NiS/CdS NWs is also proposed. During the photocatalytic HER, the photo-generated electrons can be easily transferred to the surface of β-NiS due to the intimate contact between β-NiS and CdS and the high electrical conductivity of β-NiS, thus promoting the separation of photo-generated electron–hole pairs effectively. Besides, the presence of the β-NiS co-catalyst can enhance the visible light-harvesting ability of the NiS/CdS hybrid catalyst.
The present synthesis strategy provides new insights into the design and development of high-performance heterostructured photocatalysts for solar-driven H2 evolution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 11674035, 11274052, and 61274015) and the Fund of State Key Laboratory of Information Photonics and Optical Communications (Beijing University of Posts and Telecommunications).Notes and references
- X. X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148 RSC.
- Y. Xu, Y. Huang and B. Zhang, Inorg. Chem. Front., 2016, 3, 591 RSC.
- Z. J. Sun, H. F. Zheng, J. S. Li and P. W. Du, Energy Environ. Sci., 2015, 8, 2668 CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- T. Simon, N. Bouchonville, M. J. Berr, A. Vaneski, A. Adrović, D. Volbers, R. Wyrwich, M. Döblinger, A. S. Susha, A. L. Rogach, F. Jäckel, J. K. Stolarczyk and J. Feldmann, Nat. Mater., 2014, 13, 1013 CrossRef CAS PubMed.
- F. Y. Wen and C. Li, Acc. Chem. Res., 2013, 46, 2355 CrossRef CAS PubMed.
- H. J. Yan, J. H. Yang, G. J. Ma, G. P. Wu, X. Zong, Z. B. Lei, J. Y. Shi and C. Li, J. Catal., 2009, 266, 165 CrossRef CAS.
- K. F. Wu, Z. Y. Chen, H. J. Lv, H. M. Zhu, C. L. Hill and T. Q. Lian, J. Am. Chem. Soc., 2014, 136, 7708 CrossRef CAS PubMed.
- Z. B. Yu, Y. P. Xie, G. Liu, G. Q. Lu, X. L. Ma and H. M. Cheng, J. Mater. Chem. A, 2013, 1, 2773 CAS.
- N. Z. Bao, L. M. Shen, T. Takata and K. Domen, Chem. Mater., 2008, 20, 110 CrossRef CAS.
- C. Zhu, C. G. Liu, Y. J. Zhou, Y. J. Fu, S. J. Guo, H. Li, S. Q. Zhao, H. Huang, Y. Liu and Z. H. Kang, Appl. Catal., B, 2017, 216, 114 CrossRef CAS.
- S. M. Yin, J. Y. Han, Y. J. Zou, T. H. Zhou and R. Xu, Nanoscale, 2016, 8, 14438 RSC.
- J. H. Xiong, Y. H. Liu, D. K. Wang, S. J. Liang, W. M. Wu and L. Wu, J. Mater. Chem. A, 2015, 3, 12631 CAS.
- X. Zong, H. J. Yan, G. P. Wu, G. J. Ma, F. Y. Wen, L. Wang and C. Li, J. Am. Chem. Soc., 2008, 130, 7176 CrossRef CAS PubMed.
- Q. D. Yue, Y. Y. Wan, Z. J. Sun, X. J. Wu, Y. P. Yuan and P. W. Du, J. Mater. Chem. A, 2015, 3, 16941 CAS.
- J. Z. Chen, X. J. Wu, L. S. Yin, B. Li, X. Hong, Z. X. Fan, B. Chen, C. Xue and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1210 CrossRef CAS PubMed.
- J. S. Jang, D. J. Ham, N. Lakshminarasimhan, W. Y. Choi and J. S. Lee, Appl. Catal., A, 2008, 346, 149 CrossRef CAS.
- X. Zong, J. F. Han, G. J. Ma, H. J. Yan, G. P. Wu and C. Li, J. Mater. Chem. C, 2011, 115, 12202 CAS.
- W. T. Bi, L. Zhang, Z. T. Sun, X. G. Li, T. Jin, X. J. Wu, Q. Zhang, Y. Luo, C. Z. Wu and Y. Xie, ACS Catal., 2016, 6, 4253 CrossRef CAS.
- X. Zhou, J. Jin, X. J. Zhu, J. Huang, J. G. Yu, W. Y. Wong and W. K. Wong, J. Mater. Chem. A, 2016, 4, 5282 CAS.
- L. J. Zhang, R. Zheng, S. Li, B. K. Liu, D. J. Wang, L. L. Wang and T. F. Xie, ACS Appl. Mater. Interfaces, 2014, 6, 13406 CAS.
- J. L. Yuan, J. Q. Wen, Q. Z. Gao, S. C. Chen, J. M. Li, X. Li and Y. P. Fang, Dalton Trans., 2015, 44, 1680 RSC.
- Z. J. Sun, H. L. Chen, L. Zhang, D. P. Lu and P. W. Du, J. Mater. Chem. A, 2016, 4, 13289 CAS.
- X. P. Chen, W. Chen, P. B. Lin, Y. Yang, H. Y. Gao, J. Yuan and W. F. Shangguan, Catal. Commun., 2013, 36, 104 CrossRef CAS.
- X. P. Chen, W. Chen, H. Y. Gao, Y. Yang and W. F. Shangguan, Appl. Catal., B, 2014, 152–153, 68 CrossRef CAS.
- Z. Khan, M. Khannam, N. Vinothkumar, M. De and M. Qureshi, J. Mater. Chem., 2012, 22, 12090 RSC.
- J. R. Ran, J. G. Yu and M. Jaroniec, Green Chem., 2011, 13, 2708 RSC.
- W. Zhang, Y. B. Wang, Z. Wang, Z. Y. Zhong and R. Xu, Chem. Commun., 2010, 46, 7631 RSC.
- J. Zhang, S. Z. Qiao, L. F. Qi and J. G. Yu, Phys. Chem. Chem. Phys., 2013, 15, 12088 RSC.
- Z. X. Qin, Y. B. Chen, X. X. Wang, X. Guo and L. J. Guo, ACS Appl. Mater. Interfaces, 2016, 8, 1264 CAS.
- Z. X. Qin, Y. B. Chen, Z. X. Huang, J. Z. Su, Z. D. Diao and L. J. Guo, J. Phys. Chem. C, 2016, 120, 14581 CAS.
- L. J. Zhang, T. F. Xie, D. J. Wang, S. Li, L. L. Wang, L. P. Chen and Y. C. Lu, Int. J. Hydrogen Energy, 2013, 38, 11811 CrossRef CAS.
- Z. J. Sun, Q. D. Yue, J. S. Li, J. Xu, H. F. Zheng and P. W. Du, J. Mater. Chem. A, 2015, 3, 10243 CAS.
- X. Yang, L. Zhou, B. Yang, X. Q. Zuo, G. Li, A. L. Feng, H. B. Tang, H. J. Zhang, M. Z. Wu, Y. Q. Ma, S. W. Jin, Z. Q. Sun and X. S. Chen, J. Electrochem. Soc., 2014, 161, 711 CrossRef.
- Y. Pan, Y. J. Chen, X. Li, Y. Q. Liu and C. G. Liu, RSC Adv., 2015, 5, 104740 RSC.
- G. R. Yang, W. Yan, Q. Zhang, S. H. Shen and S. J. Ding, Nanoscale, 2013, 5, 12432 RSC.
- F. X. Xiao, J. W. Miao, H. B. Tao, S. F. Hung, H. Y. Wang, H. B. Yang, J. Z. Chen, R. Chen and B. Liu, Small, 2015, 11, 2115 CrossRef CAS PubMed.
- S. C. Yan, L. T. Sun, P. Qu, N. P. Huang, Y.C. Song and Z. D. Xiao, J. Solid State Chem., 2009, 182, 2941 CrossRef CAS.
- A. F. Alkaim, T. A. Kandiel, F. H. Hussein, R. Dillert and D. W. Bahnemann, Appl. Catal., A, 2013, 466, 32 CrossRef CAS.
- J. Y. Xue, W. L. Ma, L. Wang and H. T. Cui, J. Sol-Gel Sci. Technol., 2016, 78, 120 CrossRef CAS.
- S. C. Yan, Y. Shi, L. T. Sun, Z. D. Xiao, B. Sun and X. Xu, Mater. Sci. Eng., B, 2013, 178, 109 CrossRef CAS.
- H. W. Xu, J. Brito and O. A. Sadik, J. Electrochem. Soc., 2003, 150, 816 CrossRef.
- H. W. Nesbitt, D. Legrand and G. M. Bancroft, Phys. Chem. Miner., 2000, 27, 357 CrossRef CAS.
- M. Luo, Y. Liu, J. C. Hu, H. Liu and J. L. Li, ACS Appl. Mater. Interfaces, 2012, 4, 1813 CAS.
- J. Zhang, L. F. Qi, J. R. Ran, J. G. Yu and S. Z. Qiao, Adv. Energy Mater., 2014, 4, 1301925 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: SEM, TEM, and EDX examinations, XRD identification, and photocatalytic evaluation of additional samples. Comparison of the photocatalytic performances of photocatalysts reported in the literature. See DOI: 10.1039/c7sc03928j |
| This journal is © The Royal Society of Chemistry 2018 |