Polyunsaturated fatty acid amides from the Zanthoxylum genus – from culinary curiosities to probes for chemical biology
Jason J.
Chruma
 *a,
Douglas J.
Cullen
b,
Lydia
Bowman
b and
Patrick H.
Toy
*a,
Douglas J.
Cullen
b,
Lydia
Bowman
b and
Patrick H.
Toy
 *b
*b
aKey Laboratory of Green Chemistry & Technology (MOE), College of Chemistry, Sino-British Materials Research Institute, College of Physical Science & Technology, Sichuan University, No. 29, Wangjiang Road, Chengdu, Sichuan 610064, P. R. China. E-mail: chruma@scu.edu.cn
bDepartment of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China. E-mail: phtoy@hku.hk
First published on 4th January 2018
Abstract
Covering up to February 2017
The pericarps of several species from the Zanthoxylum genus, a.k.a. the “prickly ash”, have long been used for culinary purposes throughout Asia, most notably in the Sichuan (previously Szechuan) cuisine of Southwestern China, due to the unique tingling and numbing orosensations arising from a collection of polyunsaturated fatty acid amide (alkamide) constituents. The past decade has experienced dramatically increased academic and industrial interest in these pungent Zanthoxylum-derived alkamides, with a concomitant explosion in studies aimed at elucidating the specific biochemical mechanisms behind several medically-relevant biological activities exhibited by the natural products. This rapid increase in interest is partially fueled by advances in organic synthesis reported within the past few years that finally have allowed for the production of diastereomerically-pure Zanthoxylum alkamides and related analogs in multigram quantities. Herein is a comprehensive review of the discovery, total synthesis, and biological evaluation of Zanthoxylum-derived polyunsaturated fatty acid amides and synthetic analogues. Critical insights into how chemical synthesis can further benefit future chemical biology efforts in the field are also provided.
1. Introduction
In essentially every quadrant of the globe, local cultures have made use of plants from the Zanthoxylum genus (a.k.a. “prickly ash” or “toothache tree”) of the Rutaceae family. Extracts from the bark and berries of Z. clava-herculis (Southern prickly ash) and Z. americanum (Northern prickly ash) have long been incorporated into traditional Native American herbal remedies.1 Likewise, several Zanthoxylum species are essential components in traditional Nigerian medicine for the treatment of a wide range of conditions.2 The pulverized peppercorns from the Japanese Z. piperitum, referred to locally as sanshō ( ), are both an essential culinary spice for broiled eel (kabayaki unagi) and a vital component in a traditional Japanese therapy (Kampo) for treating digestive disorders.3,4 Most notably, the pericarps from the Chinese species Z. bungeanum (red huajiao,
), are both an essential culinary spice for broiled eel (kabayaki unagi) and a vital component in a traditional Japanese therapy (Kampo) for treating digestive disorders.3,4 Most notably, the pericarps from the Chinese species Z. bungeanum (red huajiao,  ) and Z. schinifolium (green huajiao or tengjiao,
) and Z. schinifolium (green huajiao or tengjiao,  ) are synonymous with the cuisine of Sichuan (formerly Szechuan) Province, collectively being referred to internationally as “Sichuan peppercorn”.5 This universal interest for both culinary and medicinal applications is a direct result of a variety of predominantly pungent polyunsaturated fatty acid amides (alkamides), several of which are produced exclusively by members of the Zanthoxylum genus. These polyunsaturated fatty acid amide natural products have long held the interest of the synthetic organic community, but it has only been within the past few years that procedures were described for the multigram-scale synthesis of cis-olefin-containing Zanthoxylum alkamides as single diastereomers. Likewise, concerted international efforts toward the exploration of Zanthoxylum alkamides and related synthetic analogues as chemical tools to probe and elucidate biological processes and activities are predominantly confined within the last decade. In contrast to previous reports surveying Zanthoxylum natural products6 or alkamides in general,7 this review aims to be a comprehensive collection of the discoveries, syntheses and biological evaluations of Zanthoxylum-derived alkamides and synthetic analogues (up through early 2017). Critical insight as to how chemical synthesis can further advance biomolecular studies of these natural products also will be provided.
) are synonymous with the cuisine of Sichuan (formerly Szechuan) Province, collectively being referred to internationally as “Sichuan peppercorn”.5 This universal interest for both culinary and medicinal applications is a direct result of a variety of predominantly pungent polyunsaturated fatty acid amides (alkamides), several of which are produced exclusively by members of the Zanthoxylum genus. These polyunsaturated fatty acid amide natural products have long held the interest of the synthetic organic community, but it has only been within the past few years that procedures were described for the multigram-scale synthesis of cis-olefin-containing Zanthoxylum alkamides as single diastereomers. Likewise, concerted international efforts toward the exploration of Zanthoxylum alkamides and related synthetic analogues as chemical tools to probe and elucidate biological processes and activities are predominantly confined within the last decade. In contrast to previous reports surveying Zanthoxylum natural products6 or alkamides in general,7 this review aims to be a comprehensive collection of the discoveries, syntheses and biological evaluations of Zanthoxylum-derived alkamides and synthetic analogues (up through early 2017). Critical insight as to how chemical synthesis can further advance biomolecular studies of these natural products also will be provided.
2. Isolation and characterization
2.1. Sanshools and hydroxy-sanshools
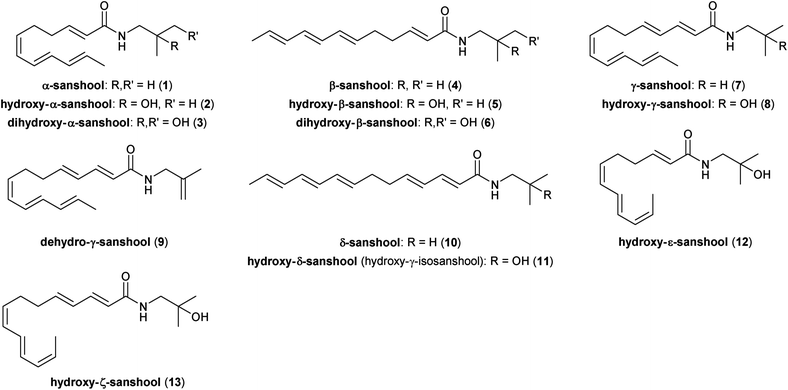
The sanshools are the predominant class of long-chained polyunsaturated amides produced by the Zanthoxylum genus (Fig. 1). These alkamide natural products are characterized by the presence of three conjugated double bonds at the Δ-end of the fatty acid chain. The nomenclature of the sanshools is such that the term “sanshool” refers to the non-functionalized isobutylamide group, with “hydroxy-sanshool” referring to the (2-hydroxy)isobutylamide congeners.Although the pungent components found in the Zanthoxylum genus had previously been investigated chemically,8,9 it was not until 1955 that the first sanshool structure, α-sanshool (1, previously referred to as either echinacein10 or neoherculin11,12) was correctly identified as (2E,6Z,8E,10E)-N-(isobutyl)dodeca-2,6,8,10-tetraenamide.11,12 While most commonly associated with the Zanthoxylum genus, alkamide 1 also has been isolated from Echinacea angustifolia.10 Crombie and Tayler later reported the successful extraction and isolation of 1 from Z. piperitum DC and a subsequent stereomutation to form β-sanshool (4), as well as confirming the respective stereochemistries of each polyene.13 Longer chain sanshools were reported later. γ-Sansool (7) and hydroxy-γ-sanshool (8) were both first isolated from the pericarps of Z. ailanthoides by Yasuda and co-workers.14 Yasuda's group was particularly active in the field of sanshool research as they were also the first to report the isolation of hydroxy-α-sanshool (2)15 and hydroxy-β-sanshool (5).16 Moreover, they determined that the all-(E) polyene 5 could be generated from the cis-alkene 2 by irradiation of the latter with UV light in the presence of iodine.16 The distribution of the alkamides within the plant suggests that the hydroxylation of the isobutylamine region occurs within the fruit, whereas the sanshool compounds are present throughout the plant, particularly in the bark.15–17 These hydroxylated variants, particularly 2, are mostly unique to the Zanthoxylum genus. The corresponding all-(E) C14-isomer, hydroxy-δ-sanshool (11, occasionally referred to as hydroxy-γ-isosanshool)18–20 was isolated in 1988,13 and the dehydroxylated congener δ-sanshool (10) was reported nine years later.14 Derivatives of the sanshools with distinct functional variations have also been reported, including dihydroxylated derivatives of α- and β-sanshool (3 & 6)20–23 and a dehydrated derivative of hydroxy-γ-sanshool, dehydro-γ-sanshool (9).23,24 Bryant and Mezine described hydroxy-ε-sanshool (12) when conducting investigations into the neurological activity of the sanshool family.25 The structure of this substance was proposed by comparison with data published independently by the groups of both Yasuda15 and Kashiwada.26 Alkamide 12 also was proposed as a product of isolation in 2008,21 once again using comparison of spectra to those reported in the Yasuda and Kashiwada papers. A conclusive isolation and structural analysis for 12, however, was not reported until 2014 by Bader and co-workers,18 who noted that 12 was not, in fact, reported by either Yasuda or Kashiwada. Bader's group was also responsible for the isolation from Z. piperitum pericarps (using supercritical fluid extraction) and structural elucidation of the rare and unstable hydroxy-ζ-sanshool (13) in the same report.
2.2. Bungeanools
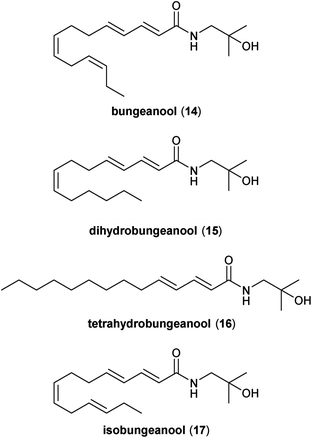
Dried pericarps from Z. bungeanum MAXIM, referred to locally as red huajiao ( ) and internationally as Sichuan peppercorn, are ubiquitous in authentic Sichuan cuisine due to their distinctive taste and the unique numbing and tingling sensations that they impart from the collection of alkamides present.5 As the name suggests, the bungeanools were first isolated from the pericarps of this particular Zanthoxylum species (Fig. 2). Structurally, the bungeanools are essentially partially saturated variants of hydroxy-γ-sanshool (8). The first extraction of the bungeanools was achieved by successive silica gel column chromatography, followed by HPLC purification of a chloroform extract of fresh Z. bungeanum pericarps.16 In addition to hydroxy-sanshools 2, 4, 7, and 11, this procedure also afforded two new alkamides as unstable colourless syrups. Specifically, the (E,E,Z,Z)-tetraene bungeanool (14) and the (E,E,Z,E)-diastereomer isobungeanool (17) were isolated in 0.016 and 0.008% yields, respectively. These alkamide natural products were also recovered, along with two new compounds, dihydrobungeanool (15) and tetrahydrobungeanool (16), from 95% ethanolic extracts of the air-dried pericarps of the same species.24 Methanolic extracts of Z. bungeanum pericarps have also yielded 14.27
) and internationally as Sichuan peppercorn, are ubiquitous in authentic Sichuan cuisine due to their distinctive taste and the unique numbing and tingling sensations that they impart from the collection of alkamides present.5 As the name suggests, the bungeanools were first isolated from the pericarps of this particular Zanthoxylum species (Fig. 2). Structurally, the bungeanools are essentially partially saturated variants of hydroxy-γ-sanshool (8). The first extraction of the bungeanools was achieved by successive silica gel column chromatography, followed by HPLC purification of a chloroform extract of fresh Z. bungeanum pericarps.16 In addition to hydroxy-sanshools 2, 4, 7, and 11, this procedure also afforded two new alkamides as unstable colourless syrups. Specifically, the (E,E,Z,Z)-tetraene bungeanool (14) and the (E,E,Z,E)-diastereomer isobungeanool (17) were isolated in 0.016 and 0.008% yields, respectively. These alkamide natural products were also recovered, along with two new compounds, dihydrobungeanool (15) and tetrahydrobungeanool (16), from 95% ethanolic extracts of the air-dried pericarps of the same species.24 Methanolic extracts of Z. bungeanum pericarps have also yielded 14.27
The pericarps from Z. schinifolium are also used quite extensively in Sichuan cuisine. One very common use for these peppercorns, as well as those from Z. bungeanum, is to extract their flavour components and pungent alkamides into edible oils. Methanol extracts from 16 commercial variants of these fragrant and numbing oils all afforded bungeanools 14, 16, and 17.19 Members of the bungeanool family have also been extracted from other Zanthoxylum species such as Z. integrifoliolum.28
2.3. Lanyuamides
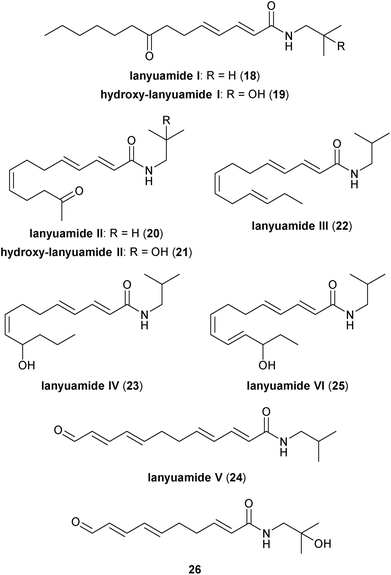
The lanyuamides are another family of Zanthoxylum alkamides closely related in structure to those previously mentioned (Fig. 3). All six members of the lanyuamide family reported to date consist of a 12- or 14-carbon unsaturated (and frequently oxidized) fatty acid chain generally linked to isobutylamine via an amide bond. The initial discovery of the lanyuamides, specifically lanyuamides I–III (18, 20, 22) originated from a methanolic extract of the dried fruits of Z. integrifoliolum.28 While lanyuamide III (22) is effectively the dehydroxylated variant of isobungeanool (17), lanyuamides I and II (18 and 20, respectively) represent the first reported examples of ketone-containing Zanthoxylum-derived alkamides. In a following report, the three related partially-oxidized polyunsaturated fatty acid isobutyl amides lanyuamides IV–VI (23–25) were isolated and characterized from a methanolic extract of the dried flowers of Z. integrifoliolum.29 Aldehyde 24 is the only member of the lanyuamides that possesses a C12 fatty acid component and resembles the product of oxidative cleavage of the terminal olefin in δ-sanshool (10). In 2012, Zhou and co-workers isolated a similar as-of-yet unnamed aldehyde-containing C10 alkamide (26) from Z. bungeanum pericarps.27 The remaining two members of the lanyuamides, 23 and 25, contain a single alcohol moiety. Both 23 and 25 possess one chirality centre and they exhibit levorotatory optical activity, but the exact stereochemical configurations for these two alkamides have not been assigned conclusively. While it is known that the sanshools and bungeanools are sensitive to both alcoholic solvents and oxidation of the fatty acid chain,30 the lanyuamides appear to be genuine natural products and not artefacts of the isolation process. The (2-hydroxy)isobutylamide variants of lanyuamide I and lanyuamide II also have been reported.31 Specifically, hydroxylated alkamides 19 and 21 were isolated as colourless gels from a methanolic extract of the root bark of Z. ailanthoides, which is sometimes referred to as “Japanese prickly ash”.2.4. ZP-amides
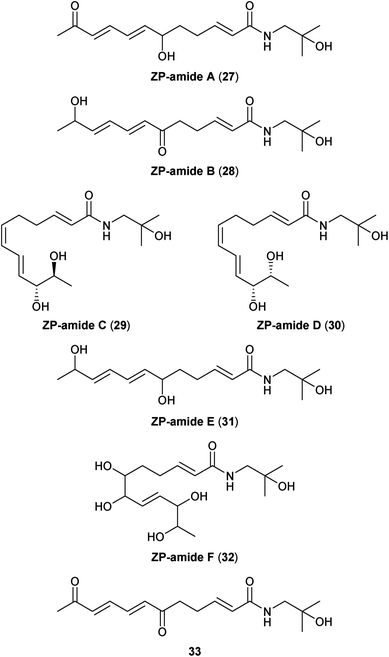
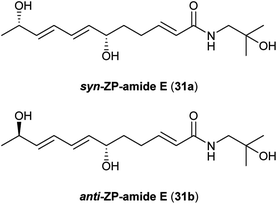
Just as the lanyuamides can be described as partially oxidized versions of the C14-fatty acid chain-containing γ- and δ-sanshools, the ZP-amides exhibit a similar relation to the C12 alkamides hydroxy-α-sanshool and hydroxy-β-sanshool (Fig. 4). ZP-amides A–E (27–31) were first reported by Hatano and co-workers as unstable colourless syrups isolated from the pericarps of Z. piperitum fruits;32 while not clearly stated by the authors, it is most likely that the “ZP” in the names for these compounds refers to the source species. Even though all of the ZP-amides isolated in this original report (27–31) contain at least one stereogenic centre, none of them exhibit any optical activity suggesting that they all were isolated as racemic compounds. The relative stereochemistry of the syn-diol unit in ZP-amide D (30) was determined by treatment with acetone and analysis of the resulting dimethyl ketal by rotating-frame Overhauser spectroscopy (ROESY). The relative stereochemistry in anti-diol ZP-amide C (29) was thus established by comparison to the syn-diol 30. In the original isolation paper, Hatano and co-workers isolated ZP-amide E (31) as two distinct racemic compounds, presumably syn and anti diastereomers, and labelled them collectively as ZP-amides E and F. Unfortunately, later reports by other researchers in the field simply referred to these two diastereomeric compounds collectively as “ZP-amide E”.33–35 To further complicate matters, Kim and co-workers isolated a new pentahydroxylated member of the ZP-amide family (32) as a colourless wax from Z. piperitum pericarps and titled it ZP-amide F,34 without recognizing that Hatano and co-workers had already used this name to refer to one of the diastereomers of 31. To alleviate this conflict in the literature, we propose that the two possible diastereostereomers for compound 31 be referred to as syn-ZP-amide E (31a) and anti-ZP-amide E (31b), as shown in Fig. 5, and the polyhydroxylated alkamide 32 reported by Kim and co-workers retain the name ZP-amide F. Unlike other members of the ZP-amide family, polyol 32 did exhibit modest optical activity, suggesting that it is not a racemic mixture. Unfortunately, the relative and absolute configuration of this unique alkamide has yet to be established conclusively. Finally, in addition to ZP-amide A, Huang and co-workers reported that the related unnamed diketone 33 could be isolated from the dried pericarps of Z. bungeanum.272.5. Qinbunamides
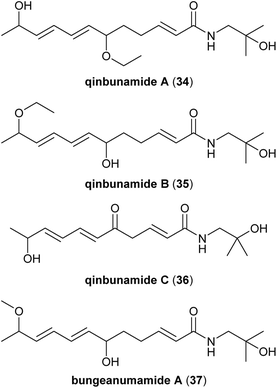
As part of a recent campaign to discover unique natural products capable of enhancing neurotrophic activity, the groups of Zhang and Gao screened isolates from ethanolic extracts of Z. bungeanum pericarps and accordingly introduced what might be the most controversial members of the Zanthoxylum alkamide family: the qinbunamides (Fig. 6).35 As with ZP-amides A–E, all three members of the qinbunamides contain at least one chirality centre but lack any optical activity, suggesting that they were isolated as racemic mixtures. Qinbunamide A (34) and qinbunamide B (35) are unique in that they both contain a rare ethyl ether moiety. Given that (1) the sanshools are known to be unstable compounds sensitive to oxygen, light, and hydroxylated solvents30 and (2) ethanol was used as the initial extraction solvent, it is likely that 34 and 35 are artefacts of the isolation process. In the original report, the authors provide HPLC traces of the resulting extraction mixtures when either ethanol or a different solvent (chloroform) were used for isolation of the alkamides, but the peaks corresponding to 34 and 35 are not clearly identified in these traces.35 The supposition that 35 is an artefact of isolation is corroborated by reports of the related compound bungeanumamide A (37), which is the methyl ether analogue of qinbunamide B (35). Methyl ether 37 has been reported twice and in both cases, the method of isolation from Zanthoxylum pericarps was nearly identical to that for 35 except that methanol was used for the extraction instead of ethanol.34,36 Given that the methyl ether 37 was isolated when methanol is employed, but ethyl ether 35 was observed using ethanol, it is most likely that these products (34, 35, and 37) arise from a reaction between the specific hydroxylated solvent used for extraction and a common reactive precursor. Further studies are required to either support or refute this supposition. The last member of the qinbunamide family, qinbunamide C (36), is less controversial. In addition to possessing a ketone and a hydroxyl moiety, 36 is the only Zanthoxylum alkamide reported to date with a C11 fatty acid chain.2.6. Timuramides
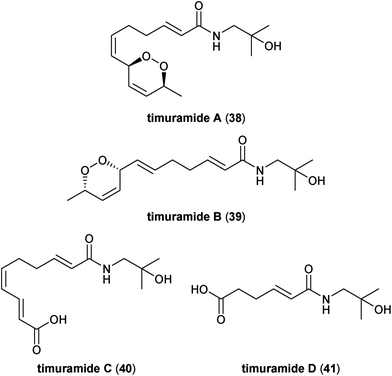
The timuramides (Fig. 7) were originally reported by Beutler and co-workers in 2013 as novel alkamides isolated from methanolic extracts of dried pericarps from Nepalese Z. armatum (referred to locally as “timur”).33 Timuramide A (38) and timuramide B (39) both possess an unusual endoperoxide group suggesting a formal [4 + 2] cycloaddition between oxygen and either hydroxy-α-sanshool (2) or hydroxy-β-sanshool (5), respectively. Both 38 and 39 exhibit optical activity, indicating that they are single enantiomers. Unfortunately, attempts to elucidate the absolute configuration of 38, such as via selective hydrogenolysis of the endoperoxide moiety and conversion of the resulting diol to the corresponding bis-Mosher's ester, did not yield fruit. The other two members of the timuramide family, timuramides C (40) and D (41), also appear to be oxidized metabolites of hydroxy-α-sanshool (2) in which either the terminal or distal alkene in the conjugated triene tail of 2 is cleaved oxidatively to the corresponding carboxylic acid, respectively. Currently, timuramide C (40) is the only member of the timuramide family to be found as a metabolite in other Zanthoxylum species, specifically from the pericarps of Korean Z. piperitum.342.7. Zanthoamides
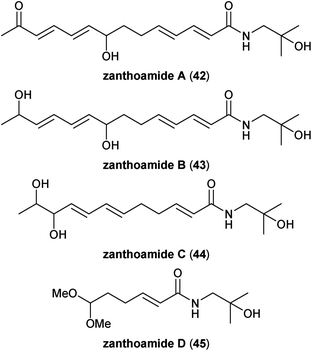
The latest addition to the family of Zanthoxylum-derived polyunsaturated fatty acid amides are the zanthoamides, reported from the groups of Wei and Gao in 2016 as products isolated from Z. bungeanum pericarps (Fig. 8).37 Zanthoamides A (42) and B (43) are essentially C14-analogues of the partially-oxidized C12-chain alkamides ZP-amide A (27) and ZP-amide E (31), respectively. Similarly, zanthoamide C (44) is the all-trans olefin variation of ZP-amides C (29) and/or D (30). Zanthoamides A–C all contain at least one stereogenic centre, but are considered naturally racemic compounds due to their lack of optical activities. The relative stereochemistries of diols 43 and 44 were not determined and could be mixtures of diastereomers, as was the case with the analogous ZP-amides. The final member of the family is the truncated alkamide zanthoamide D (45). Given the extensive use of methanol in the isolation and purification process, it is possible that the unusual dimethylacetal group in 45 is an artefact and the actual natural product is the corresponding aldehyde, representing a reduced form of carboxylic acid timuramide D (41). If this is indeed the case, then lanyuamide V (24) and trienal 26 would not be the only Zanthoxylum alkamides that possess an aldehyde moiety.2.8. Related polyunsaturated amides
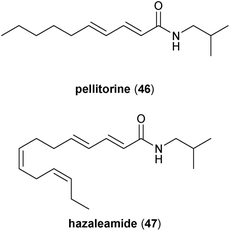
The alkamides described in the previous sections are those most associated with the Zanthoxylum genus, but these plants also produce a number of other polyunsaturated fatty acid amides common to the Rutaceae and other plant families (Fig. 9). For example, the C10 fatty acid isobutyl amide pellitorine (46), which is a metabolite found in several different plant families,38 has been isolated from the roots, pericarps, and/or stem bark from Z. tessmannii,39Z. zanthoxyloides,40Z. achtoum,41 and Z. heitzii.42,43 Additionally, the C14 tetraene alkamide hazaleamide (47), which is the dehydroxylated derivative of bungeanool 14, was first isolated from the bark of Fagara rhetza (Rutaceae), an Indonesian medicinal plant,44 but it was also extracted from the dried fruits of Taiwanese Z. integrifoliolum.28 For the sake of this review, focus will be maintained on those alkamides most associated to the Zanthoxylum genus, specifically those discussed in Sections 2.1 to 2.7.3. Synthetic studies
3.1. Early synthetic studies and challenges
The first reported chemical synthesis of any of the Zanthoxylum alkamides presented above appeared in 1969, when Sonnet described the preparation of α-sanshool (1) and its isomerization into β-sanshool (4).45 As outlined in Scheme 1, a linear synthetic route was used starting with sorbaldehyde (48). This was olefinated using a Wittig reaction with phosphonium salt 49 to produce predominantly the Z-alkene containing ester 50, which was subsequently hydrolysed to the corresponding carboxylic acid 51. Transformation of 51 into 52 was accomplished by a 4-step sequence of: (1) activation of the carboxylic acid group, (2) amidation with aziridine, (3) reduction to the corresponding aldehyde, and (4) Doebner–Miller condensation. Carboxylic acid 52 was converted into 1 (12% overall yield) by reaction with oxalyl chloride to generate the corresponding acid chloride, followed by treatment with isobutyl amine. Finally, Z-alkene 1 was converted into the corresponding E-olefin 4 in 72% yield (9% overall yield) by treatment with iodine in visible light. While successful, this work suffers from the fact that many of the compounds synthesized were reported to be mixtures of alkene isomers. Due to the limitations of analytical chemistry at the time, however, the details of these mixtures were not adequately discussed.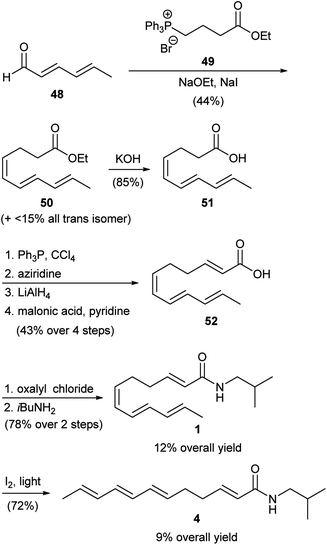 | ||
| Scheme 1 Sonnet synthesis of α-sanshool (1) and β-sanshool (4).45 | ||
Some years later, Crombie and Fisher reported the synthesis of mixtures of α-sanshool (1) and β-sanshool (4), and γ-sansool (7) and δ-sanshool (9) via routes that also relied on Wittig reactions using aldehyde 48 as a starting material (Scheme 2).46,47 The reactions using phosphonium salts 53 and 54 (the synthesis of which required numerous steps from simple starting materials) afforded mixtures of 1 and 4, and 7 and 9, respectively. Unfortunately, no discussion regarding the separation of these mixtures into their individual components was provided.
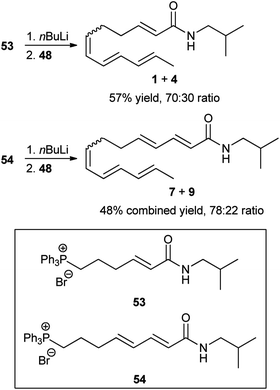 | ||
| Scheme 2 Crombie and Fisher synthesis of mixtures of α-sanshool (1) and β-sanshool (4) and γ-sanshool (7) and δ-sanshool (9).46,47 | ||
While these early synthetic studies are noteworthy, it is clear that they are, by modern standards, lacking in terms of compound characterization data provided and/or final product purity. Nowadays well characterized pure compound samples are desired for applied biological studies, and more recent synthetic efforts have been directed towards producing such sanshool samples.
3.2. Toy syntheses of sanshools and hydroxy-sanshools
Much more recently, Toy and co-workers initiated studies towards the synthesis of both the sanshools and hydroxy-sanshools. In their work, carboxylic acid synthetic intermediates that can be converted directly into either of these classes of compounds were targeted.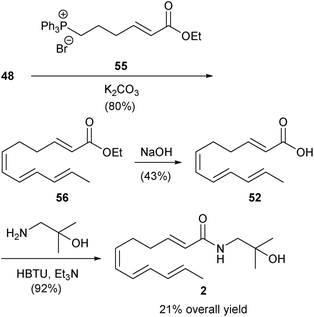 | ||
| Scheme 3 Toy group synthesis of hydroxy-α-sanshool (2).48 | ||
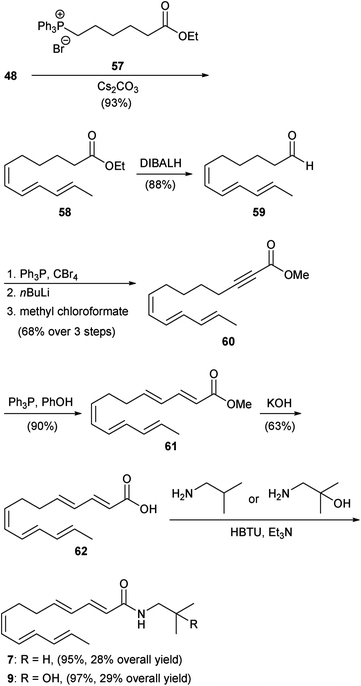 | ||
| Scheme 4 Toy group synthesis of γ-sanshool (7) and hydroxy-γ-sanshool (8).49 | ||
3.3. Igarashi syntheses of sanshools and hydroxy-sanshools
At essentially the same time as the Toy group was pursuing their studies, Igarashi and co-workers were also investigating the synthesis of some of the sanshools and hydroxy-sanshools.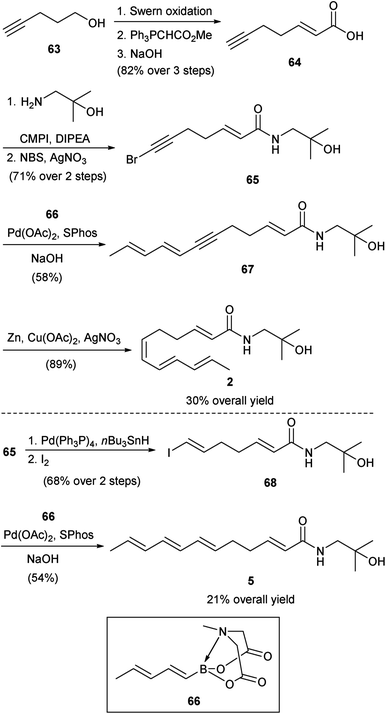 | ||
| Scheme 5 Igarashi synthesis of hydroxy-α-sanshool (2) and hydroxy-β-sanshool (5).50 | ||
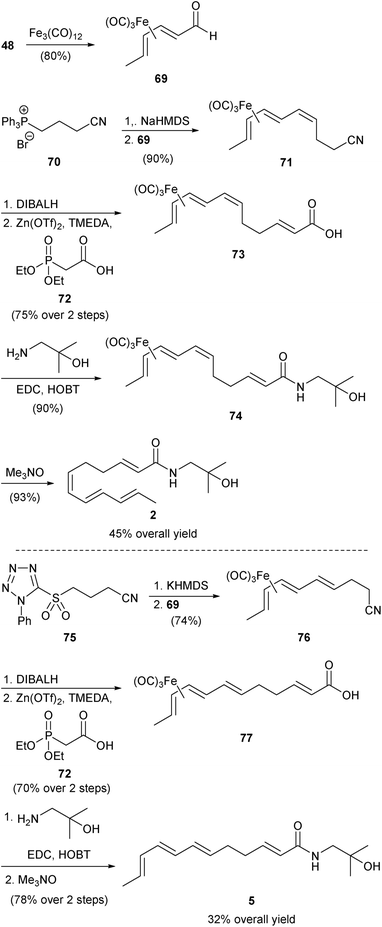 | ||
| Scheme 6 Igarashi synthesis of hydroxy-α-sanshool (2) and hydroxy-β-sanshool (5).51 | ||
As shown in Scheme 7, the synthesis of 7 was accomplished by once again reducing the nitrile group of 71 to the corresponding aldehyde followed by a HWE reaction with phosphonate 78 to afford polyene 79. Decomplexation of the iron complex in 79 produced alkamide 7 in 31% overall yield.
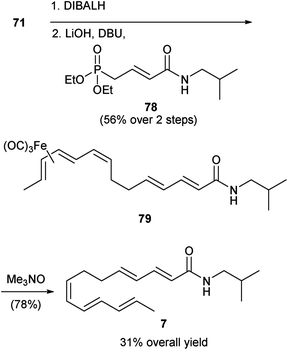 | ||
| Scheme 7 Igarashi synthesis γ-sanshool (7).51 | ||
3.4. Mugnaini syntheses of δ-sanshool
More recently, Mugnaini and Corelli described two syntheses of δ-sanshool (10).52 In their first generation synthesis they used alcohol 80 as the starting material (Scheme 8). This was first oxidized to aldehyde 81, which was subsequently subjected to a HWE olefination reaction with phosphonate 78 to afford diene 82. Deprotection of the alcohol group of 82 was followed by its oxidation to yield aldehyde 83. Finally, a second HWE olefination reaction of 83 with phosphonate 84 afforded 10 in 7% overall yield.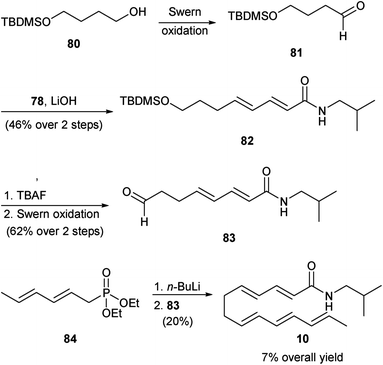 | ||
| Scheme 8 Mugnaini and Corelli synthesis of δ-sanshool (10).52 | ||
As outlined in Scheme 9, in their second generation synthesis of 10, Mugnaini and Corelli used the same building blocks, but switched the order of HWE olefination reactions used to combine them. Thus, starting aldehyde 81 was reacted with phosphonate 84 to afford (E,E,E)-triene 85. Deprotection of the alcohol group of 85 followed by its oxidation afforded intermediate aldehyde 86, which was subjected to the second HWE olefination with phosphonate 78 to produce 10 in 17% overall yield.
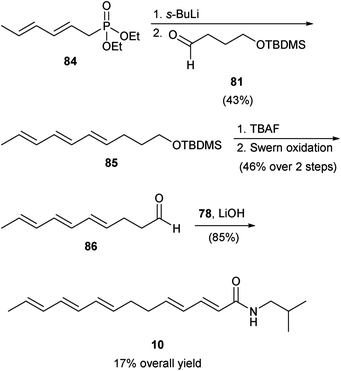 | ||
| Scheme 9 Mugnaini and Corelli second generation synthesis of δ-sanshool (10).52 | ||
3.5. Givaudan synthesis of bungeanool
The only other naturally occurring alkamide discussed above in Section 2 to be synthesized chemically is bungeanool (14). This was reported by researchers at Givaudan in 2003 and their route is outlined in Scheme 10.53 Propargyl bromide 87 was coupled with alkynol 88 to form diynol 87. Selective partial reduction of the alkyne groups of 87 to the corresponding Z-alkenes was followed by oxidation of the alcohol to afford aldehyde 90. This intermediate was then subjected to a HWE olefination reaction with phosphonate 91 to produce ester 92. Finally, 92 was converted into 14 by the reaction sequence of: (1) ester hydrolysis, (2) activation of the resulting carboxylic acid, and (3) exposure to the required amine. Unfortunately, only the yields for the formation of 92 and 14 were reported, so it is not possible to evaluate the overall efficiency of this synthesis.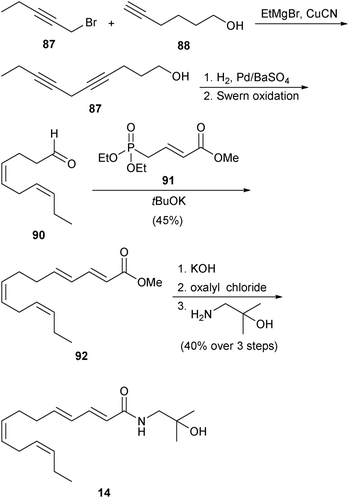 | ||
| Scheme 10 Givaudan synthesis of bungeanool (14).53 | ||
3.6. Design and synthesis of novel alkamides inspired by hydroxy-α-sanshool
In addition to the diversity of Zanthoxylum alkamides available from natural sources, a significant collection of synthetic derivatives also have been reported. The first concerted efforts in this regard were described by the group at Givaudan when they reported their synthesis of bungeanool (14).53 Specifically, they constructed a small library of synthetic alkamides related to the sanshools and bungeanools in an attempt to identify the critical structural components responsible for the pungency of these natural products (Fig. 10). The initial series of synthetic sanshool derivatives (93–98) involved truncation, elongation, or modification of the polyunsaturated tail. These compounds, coupled with natural Zanthoxylum alkamides, led to a proposal of the minimal structure required for the paresthetic (tingling) effect associated with certain hydroxy-sanshools, e.g.2 and 8. (E,E,Z)-Trieneamides 99 and 100 were then generated to test this model (see Section 4.1 for more details). Finally, a series of isobutyl and (2-hydroxy)isobutyl amides of polyunsaturated acids containing an aromatic ring (101–107) and a small collection of cinnamamides (103a–i) were constructed in the hopes of identifying a simplified and more stable alkamide with similar orosensation properties to hydroxy-α-sanshool (2).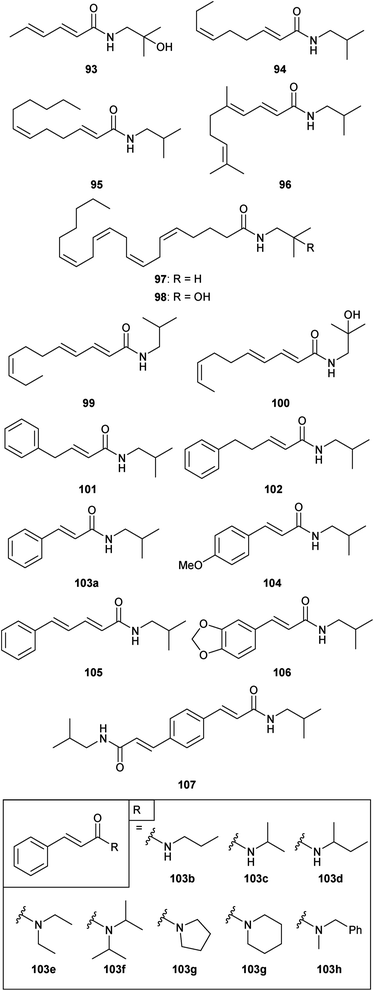 | ||
| Fig. 10 Synthetic derivatives of the sanshools and bungeanools.53 | ||
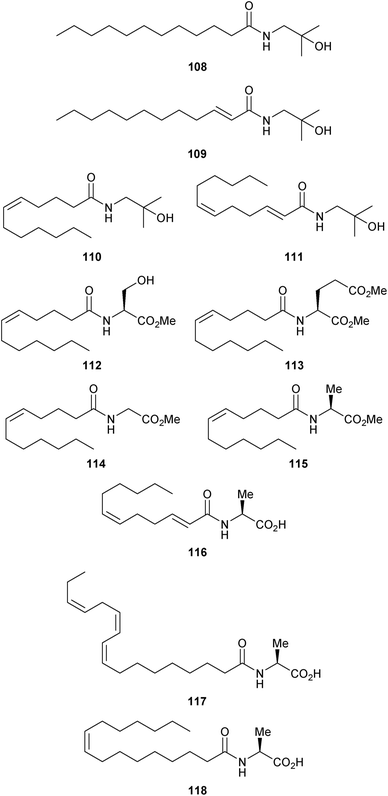
Six years after these seminal structure–activity relationship studies, researchers at Nestlé reported their own collection of synthetic hydroxy-α-sanshool analogues in an attempt to elucidate the critical structural features responsible for the compound's ability to activate transient receptor potential (TRP) channels TRPV1 and TRPA1 (see Section 4.2 for more details).54 As shown in Fig. 11, these derivatives fell into three categories: (1) (2-hydroxy)isobutyl amides of four different C12 fatty acid tails containing between zero to two double bonds (108–111), (2) amides between (Z)-dodec-5-enoic acid and either methyl L-serinate (112), dimethyl L-glutamate (113), methyl L-glycinate (114), or methyl L-alanate (115), and (3) amides between L-alanine and either (2E,6Z)-dodeca-2,6-dienoic acid (116), linolenic acid (117), or palmitoleic acid (118).
 | ||
| Fig. 11 Synthetic sanshool derivatives.54 | ||
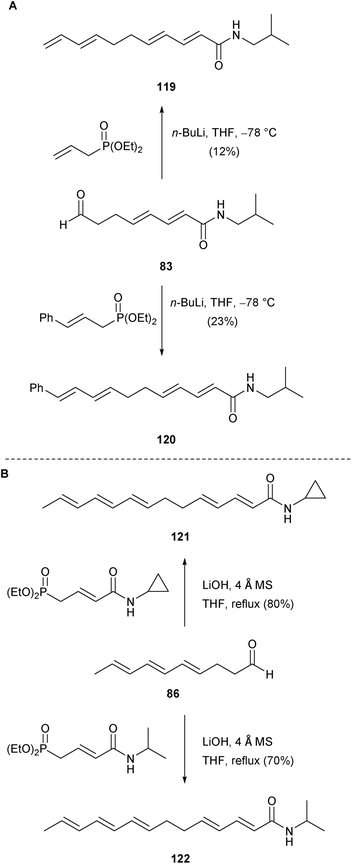
Finally, in their recent synthesis of δ-sanshool (10, see Section 3.4), Mugnaini and Corelli also generated four unique analogues of the natural product.52 Tetraeneamides 119 and 120 were both obtained in low yield by HWE olefination between the previously described aldehyde 83 and the requisite diethylphosphinates (Scheme 11A). Similarly, cyclopropyl amide 121 and isopropylamide 122 were generated via significantly higher yielding (E)-selective olefination reactions with the conjugated triene aldehyde 86 (Scheme 11B).
 | ||
| Scheme 11 (A) Synthesis of δ-sanshool tetraene analogues 119 and 120. (B) Synthesis of δ-sanshool amide derivatives 121 and 122.52 | ||
4. Biological activity and structure–activity relationships
Throughout the world, members of the Zanthoxylum genus have been incorporated into traditional medicines and herbal remedies for a wide range of medical indications. Similarly, throughout the scientific literature, a growing collection of biological effects have been attributed to Zanthoxylum-based extracts, including antioxidant, antibacterial, anticancer, anti-inflammatory and hepatoprotective. Unfortunately, there appears to be significant confusion as to the source of these observed biological activities. Several studies, particularly those associated with antibacterial activity, involve the essential oils derived from Zanthoxylum fruits;55 it is critical to emphasize that these essential oils do not contain any alkamides!30,55 Accordingly, authors are strongly encouraged to no longer attribute the biological activities observed with Zanthoxylum essential oils in previous or future studies to alkamide natural products. That being said, many of the demonstrated biological activities associated with traditional remedies containing Zanthoxylum-based components can be attributed to the pungent alkamide components present. Some of these traditional Zanthoxylum extract-based medicines are manufactured in pharmaceutical-grade and are being evaluated in modern clinical trials in both Japan and the United States, with substantial associated pharmacokinetic studies on the critical alkamide components.56–58 Cosmetic formulations involving alkamides extracted from Z. bungeanum are also under investigation for commercial usage.88 In 2016, Greger compiled a highly recommended general review of the biological activities associated with alkamide natural products from various sources.7 The following section surveys studies performed with the aim of understanding the various biological activities known to be associated specifically with Zanthoxylum alkamides. Table 1 provides an overview of the known biomolecular targets reported for different Zanthoxylum alkamides.| Alkamide | Validated cellular target (s) |
|---|---|
| Hydroxy-α-sanshool (2) | Agonistic: TRPV1,61 TPRA1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 61 |
Antagonistic: TASK-1 (KCNK3),63 TASK-3 (KCNK9),63 TRESK (KCNK18),63 CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
|
| Dihydroxy-α-sanshool (3) | Antagonistic: CB1,20 CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| Hydroxy-β-sanshool (5) | Agonistic: TRPV1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 61 |
Antagonistic: TASK-1 (KCNK3),63 CB1,20 CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
|
| Hydroxy-γ-sanshool (8) | Antagonistic: CB1,20 CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| δ-Sanshool (10) | Agonistic: CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Antagonistic: CB1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
|
| Tetrahydrobungeanool (16) | Antagonistic: CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Before discussing specific biological activities, it is worthwhile to summarize current pharmacokinetic and bioavailability data available for Zanthoxylum alkaloids. Due to the apparent hydrophobic nature of these polyunsaturated fatty acid amides, it is tempting to assume that they would only be appropriate for topical or “direct contact” applications. Current data indicates, however, that these compounds can reach therapeutically-relevant concentrations in the blood after oral ingestion, suggesting that treatment of systemic disorders is also within reach. Specifically, a pharmacokinetic analysis of the traditional Japanese medicine daikenchuto, which contains 0.25 g dried, ground Zanthoxylum piperitum (sanshō) peppercorns for every 15 g, monitored the blood concentration levels of both hydroxy-α-sanshool (2) and hydroxy-β-sanshool (5) in human subjects after oral ingestion of either 2.5 g, 5 g, or 10 g doses of the formulation.57 At the lowest dosage, blood levels of 2 and 5 reached maximum concentrations of 209 ± 100 ng mL−1 and 42.2 ± 25.5 ng mL−1, respectively, within approximately 15 minutes on average. These maximum levels increased to 664 ± 165 ng mL−1 and 131 ± 52.6 ng mL−1, respectively, at the highest dosage tested. For all three dosages investigated, the average apparent elimination half-lives for 2 and 5 were 1.66 h and 1.13 h, respectively. These in vivo studies clearly demonstrate that at least two of the Zanthoxylum alkamides are readily bioavailable and capable of systemic distribution via the bloodstream after oral administration. The relatively short elimination half-lives, however, emphasize a limitation to their usage for chronic concerns.
4.1. Orosensation
The most studied biological activity for the Zanthoxylum alkamides is their impact on orosensation. Early studies simply refer to the general property in the oral cavity as “pungency”, but at least three unique types of orosensation are now associated with Zanthoxylum alkamides: “burning”, paresthetic (tingling) and anaesthetic (numbing). This unusual tingling pungency is not unique to Zanthoxylum alkamides; spilanthol (Fig. 12), first isolated from the flowers of Acmella (Spilanthes) oleracea, as well as several related alkamides produced by plants in the genera Anacyclus, Echinacea and Heliopsis of the Asteraceae family exhibit distinct pungency.7 The Zanthoxylum alkamides, however, are the best and most completely studied members. As will be discussed in the next section, the specific biochemical mechanisms governing these unique orosensations are still hotly contested. Significant structure–activity relationship studies with naturally-produced Zanthoxylum alkamides and synthetic derivatives, on the other hand, have delineated the fundamental structural components requisite for the various orosensation activities. Prior to the work from Hofmann's group in 2014,18 orosensation studies employed solutions of Zanthoxylum alkamides or Zanthoxylum extracts containing ethanol, potentially leading to several false-negative results, particularly in regard to anaesthetic and burning properties. Nevertheless, it has long been established that one of the most abundant alkamides in Sichuan peppercorn (huajiao) and Japanese pepper (sanshō), hydroxy-α-sanshool (2), is also most responsible for the oral paresthetic sensation experienced upon consumption of these spices.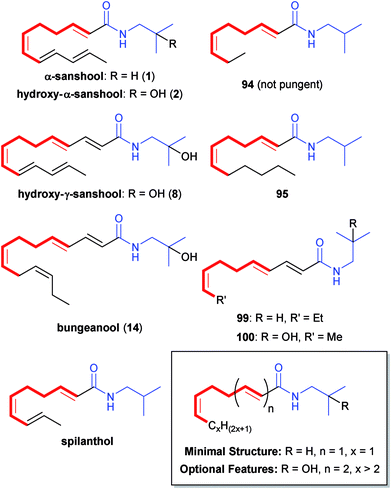 | ||
| Fig. 12 The proposed minimal structure for pungency in natural Zanthoxylum alkamides and synthetic derivatives involves an (E,Z) isolated diene unit (bold red) and an N-isobutylamide (blue). At least two of three optional features also must be present.53 | ||
In 2003, Galopin and co-workers at Givaudan were the first to propose a minimal structure required for the tingling effect,53 although the details of their orosensation studies were not provided and they simply used the qualitative descriptors of “pungent” and “not pungent” to refer to various Zanthoxylum alkamides and synthetic derivatives. Preliminary studies compared natural products α-sanshool (1), hydroxy-α-sanshool (2), hydroxy-γ-sanshool (8), bungeanool (14) and tetrahydrobungeanool (16) with synthetic derivatives 93–98 (cf.Fig. 10). From this initial collection, only alkamides 1, 2, 8, 14, and 95 proved to be “pungent”, leading the authors first to conclude that the (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Z
Z![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH2–CH
CH–CH2–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E
E![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) and N-isobutylcarboxamide motifs common to all of these compounds were most critical for the associated pungency (Fig. 12). Surprisingly, synthetic derivative 94, which possesses both of these motifs, did not demonstrate any noticeable pungency suggesting that the proposed minimal structure alone is not sufficient. Two more synthetic compounds, white powder 99 and colourless oil 100, were then synthesized to further refine the model. Both of these synthetic analogues proved to be pungent, leading the authors to conclude that for noticeable pungency the compounds must possess, in addition to the minimal structure, at least two of three optional features: (1) a hydroxyl group on the 2-position of the isobutylamide, (2) extended conjugation to the amidecarbonyl, or (3) a greater than two-carbon length chain after the critical cis olefin (Fig. 12, inset). In the hopes of identifying a simplified and stabilized analogue of α-sanshool, Galopin and co-workers also synthesized and tested a collection of aromatic alkamides (111–107, cf.Fig. 10). Unfortunately, only one compound from this series, N-isobutylcinnamamide (103a), demonstrated any noticeable pungency.
CH) and N-isobutylcarboxamide motifs common to all of these compounds were most critical for the associated pungency (Fig. 12). Surprisingly, synthetic derivative 94, which possesses both of these motifs, did not demonstrate any noticeable pungency suggesting that the proposed minimal structure alone is not sufficient. Two more synthetic compounds, white powder 99 and colourless oil 100, were then synthesized to further refine the model. Both of these synthetic analogues proved to be pungent, leading the authors to conclude that for noticeable pungency the compounds must possess, in addition to the minimal structure, at least two of three optional features: (1) a hydroxyl group on the 2-position of the isobutylamide, (2) extended conjugation to the amidecarbonyl, or (3) a greater than two-carbon length chain after the critical cis olefin (Fig. 12, inset). In the hopes of identifying a simplified and stabilized analogue of α-sanshool, Galopin and co-workers also synthesized and tested a collection of aromatic alkamides (111–107, cf.Fig. 10). Unfortunately, only one compound from this series, N-isobutylcinnamamide (103a), demonstrated any noticeable pungency.
In 2005, Sugai and co-workers evaluated the orosensation activities and tastes of four sanshools (α-, β-, γ-, and δ-) and two hydroxy-sanshools (α- and β-), all of which were isolated and purified from dried Z. piperitum pericarps.59 Unlike previous studies,53 these efforts distinguished between the capsaicin-like burning pungencies (even assigning Scoville unit values for each compound) and the numbing and tingling oral sensations; results from these investigations are summarized in Table 2. A key and unique distinction from these investigations is that the pungency of the N-isobutylamide sanshools (1, 4, 7, 10) is significantly characterized as “burning”, whereas the hydroxylated congeners (2 and 5) are exclusively tingling and/or numbing. It should be noted, however, that the burning sensation associated with the sanshools is still significantly less than that experienced from capsaicin (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 SU). Another noteworthy observation from this investigation is that, contrary to previous studies that simply identify β-sanshool (4) and hydroxy-β-sanshool (5) as “not pungent”,53 these two all-trans alkamides were noted to exhibit anaesthetic activity.
000 SU). Another noteworthy observation from this investigation is that, contrary to previous studies that simply identify β-sanshool (4) and hydroxy-β-sanshool (5) as “not pungent”,53 these two all-trans alkamides were noted to exhibit anaesthetic activity.
| Alkamide | Oral sensation | SU (mL g−1) | Taste |
|---|---|---|---|
| a In this circumstance, the Scoville unit (SU) values refer to the volume (mL) of sugar water required to dilute 1 g of the indicated alkamide so that a majority of trained tasters no longer perceive any burning pungency. Currently, no such scale is commonly recognized for measuring tingling and numbing pungencies. | |||
| α-Sanshool (1) | Burning, tingling, numbing | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
n.d. |
| β-Sanshool (4) | Numbing | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Bitter |
| γ-Sanshool (7) | Burning, numbing | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fresh, bitter |
| δ-Sanshool (10) | Burning, numbing | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fresh |
| Hydroxy-α-sanshool (2) | Tingling, numbing | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
n.d. |
| Hydroxy-β-sanshool (5) | Numbing, astringent | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Bitter |
The most thorough and reliable orosensation studies on Zanthoxylum alkamides are those reported by Bader and co-workers in 2014.18 Notable aspects from these investigations are (1) a modified “half-tongue” test60 was employed in which the compounds were administered to the tongues of the tasting panels by pre-absorption onto a square of filter paper instead of as a solution containing ethanol, thus minimizing false-negative results, and (2) tingling (paresthetic) and numbing (anaesthetic) orosensory recognition for each alkamide examined was quantitated by establishing threshold concentrations. For these studies, eight pure Zanthoxylum alkamides were obtained by supercritical fluid extraction from dried Z. piperitum pods followed by extensive HPLC purification. Specifically, the pungencies of hydroxy-α-sanshool (2), hydroxy-β-sanshool (5), hydroxy-γ-sanshool (8), hydroxy-δ-sanshool (11, referred to as hydroxy-γ-isosanshool), hydroxy-ε-sanshool (12), hydroxy-ζ-sanshool (13), bungeanool (14), and isobungeanool (17) were all explored; the results from these investigations are summarized in Table 3. All six of the Zanthoxylum alkamides investigated that possessed the critical structural motifs proposed by Galopin and co-workers (2, 8, 12–14, 17)53 elicited pronounced and roughly equipotent tingling oral sensations. In accord with the previous report from Sugai and co-workers,59 the sensory panellists all reported a potent and persistent numbing/anaesthetic oral impression from the all-trans alkamides 5 and 11. This combination of potent paresthetic (from cis-olefin containing N-(2-hydroxy)isobutyl alkamides) and anaesthetic (from the all-trans polyene congeners) activities adequately characterizes the unique tingling and numbing orosensation experienced upon eating fresh Sichuan peppercorns. Given that the paresthetic hydroxy-α-sanshool (2) is known to isomerize to the anaesthetic all-trans tetraene 5 over time, especially in the presence of UV light,16,30 this observation could also serve as an explanation as to why aged Sichuan peppercorns tend to lose their unique mouth-tingling capabilities while maintaining their tongue-numbing characteristics.
| Alkamide | Oral sensation | Threshold concentration |
|---|---|---|
| Hydroxy-α-sanshool (2) | Paresthetic | 8.3 nmol cm−2 |
| Hydroxy-β-sanshool (5) | Anaesthetic | 3.9 nmol cm−2 |
| Hydroxy-γ-sanshool (8) | Paresthetic | 6.8 nmol cm−2 |
| Hydroxy-δ-sanshool (11) | Anaesthetic | 7.1 nmol cm−2 |
| Hydroxy-ε-sanshool (12) | Paresthetic | 4.2 nmol cm−2 |
| Hydroxy-ζ-sanshool (13) | Paresthetic | 7.0 nmol cm−2 |
| Bungeanool (14) | Paresthetic | 3.5 nmol cm−2 |
| Isobungeanool (17) | Paresthetic | 3.5 nmol cm−2 |
4.2. Excitation of sensory neurons
The first reported studies aimed at determining the peripheral basis for the tingling paresthesia induced by Zanthoxylum alkamides were described by Bryant and Mezine of the Monell Chemical Senses Center.25 They found that hydroxy-α-sanshool (2) altered the levels of spontaneous activity in cool-sensitive fibres in addition to inducing activity in tactile fibres, cold nociceptors and silent fibres that were insensitive to innocuous thermal or tactile stimuli. Furthermore, 2 induced tactile or thermal sensitivity in fibres that were initially insensitive to cooling or touch. The neuronal distribution of sensitivities to capsaicin and to 2 indicated that 2 affects neurons mediating innocuous sensations, and therefore it could indeed be useful as a model stimulus for paresthesia studies.Later Oh and co-workers reported that 2 activates transient receptor potential ankyrin 1 (TRPA1) and TRP vaniloid 1 (TRPV1) ion channels in human embryonic kidney (HEK) cells.61 Specifically, 2 caused Ca2+ influx in cells transfected with TRPV1 or TRPA1, and evoked inward currents. Furthermore, 2 evoked licking behaviour when injected into a hind paw of wild-type mice, but this effect was reduced in TRPV1-deficient mice. Thus, they concluded that TRPA1 and TRPV1 are the molecular targets of 2 in sensory neurons and that activation of these ion channels explain its pungent and tingling properties. These findings provided credence to the earlier assertion that TRPV1 activation alone could not explain the properties of 2.59
This research was subsequently supported by the findings of le Coutre and his collaborators.62 They performed some simple structure–activity relationship studies and found that the Z-configuration of double bond at position C-6 of 2 was essential for its TRPA1 activation ability. Interestingly, no such structural requirements were found for TRPV1 activation. Additionally, they reported that activation of TRPA1 by 2 seemed to involve covalent bonding, but TRPV1 did not.
These ideas were later disputed by Julius and co-workers, who found that although 2 is an agonist of both TRPA1 and TRPV1, it binds to the two-pore potassium channels TASK-1, TASK-3 and TRESK (previously known as KCNK3, KCNK9 and KCNK18, respectively).63 Neuron activation through a unique mechanism involving inhibition of these pH- and anaesthetic-sensitive channels was their suggested cause of the tingling effect.
These neurophysiological studies used animal- and human-derived models to demonstrate that 2 induces bursts of neuronal firing in specific cutaneous afferents through chemical response within the receptor membrane. Stucky and Bautista, and their co-workers used an ex vivo skin-nerve preparation to examine the pattern and intensity with which the sensory terminals of cutaneous neurons respond to 2, and their findings suggested that it excites a distinct subset of D-hair, ultrasensitive light-touch receptors in the skin and targets uncharacterized populations of Aδ- and C-fibre nerve afferents.64 This research indicates that mechanosensation, or tactile response, is also integral to the effect. The response was observed in animals lacking TRPA1 and TRPV1 receptors, which suggests that neither of these mediates the effects of 2. Inhibition of both Aδ-mechanonociceptors and the activity of multiple voltage-gated sodium channel subtypes, such as Nav 1.7, by 2 is significant since the former mediates both sharp and acute pain and inflammatory pain, and the latter is a key mediator of inflammatory mechanical pain in “fast pain” mechanosensory neurons.65
These studies focused on the molecular receptor mechanisms and did not directly assess how chemical receptor events could relate to the tingling effect, and it remained unclear which fibres are responsible. To address this issue Hagura and co-workers investigated the somatosensory perception of tingling caused by Sichuan pepper in humans to identify the characteristic temporal frequency and compared this to the established selectivity of tactile afferents.66 When Sichuan pepper was applied to the lower lip of study participants, they judged the frequency of the tingling sensation on the lips by comparing this with the frequencies of mechanical vibrations applied to their right index finger. The frequency perceived of the tingling by the participants was routinely at around 50 Hz, corresponding to the range of tactile rapidly adapting 1 (RA1) afferent fibres. Furthermore, adaptation of the RA1 channel by prolonged mechanical vibration reduced the tingling frequency caused by Sichuan pepper, and this confirmed that the frequency-specific tactile channel is shared between mechanical vibration and Sichuan pepper.
More recently the same group used a perceptual interference paradigm to show that the Sichuan pepper-induced RA input indeed contributes to the human tactile processing.67 The absolute detection thresholds for vibrotactile input were measured with and without Sichuan pepper application on the fingertip, and it was found to significantly impair detection of vibrations at 30 Hz (RA channel dominant frequency), but it did not affect detection of vibrations at 240 Hz (Pacinian-corpuscle (PC) channel dominant frequency), or vibrations at 1 Hz (slowly adapting 1 (SA1) channel dominant frequency). In total these results indicate that Sichuan pepper induces a peripheral RA channel activation that is important for tactile perception, and such anomalous activation of RA channels may contribute to the tingling experience of Sichuan pepper.
4.3. Impact on gut motility
As anyone who has indulged in a traditional Sichuan-style hot pot meal is well aware, consumption of Zanthoxylum pericarps (e.g., Sichuan peppercorns) can dramatically increase gut motility and colonic activity. Indeed, pericarps from Z. piperitum (Japanese pepper or sanshō) are one of three herbal ingredients (the other two being ginger and ginseng) in the traditional Japanese medicine daikenchuto, which has been used for centuries for the treatment of various gastro-instestinal issues.68,69 TU-100 (sometimes referred to as TJ-100),57,70 a pharmaceutical-grade daikenchuto formulation, is currently under investigation by the Japanese Foundation for Multidisciplinary Treatment of Cancer and the US Food and Drug Administration in patients with postoperative ileus and ischemic intestinal disorders.67,71,72 Substantial evidence points to the Zanthoxylum alkamides hydroxy-α-sanshool (2) and, to a lesser extent, hydroxy-β-sanshool (5) as the primary components in daikenchuto responsible for the contraction of gut smooth muscle cells, induction of colonic motor activity, and increased defecation rates.73–76 Additionally, γ-sanshool (7) has been shown to improve intestinal transit in a mouse model of postoperative ileus.77 Interestingly, all three of these alkamides appear to exert their effects by different mechanisms. For example, Tsuchiya and co-workers demonstrated that 7 induced increase gut motility by serving as an agonist for TRPA1.77 Kubota and co-workers, on the other hand, revealed that colonic contraction induced by 2 was not a result of stimulation of TRPA1 or TRPV1, but most likely via blockade of the two-pore domain potassium channel KCNK9 (TASK-3).75 The colonic motor pattern induced by alkene isomer 5 was different from that observed with 2. Both 5 and 2 are antagonists for KCNK3 (TASK-1), but only 2 can block KCNK9, in addition to KCNK18 (TRESK), providing a potential explanation for the difference in observed impact on specific colonic contraction behaviour. As mentioned above, both 2 and 5 exhibit substantial dose-dependent plasma concentrations after oral administration.57,58 Additionally, direct application of these alkamides can evoke site-specific contractions within the digestive tract,69,70 suggesting two potential pathways that these alkamides can interact with the milieu of cell types and tissues involved in the digestive process.4.4. Nerve growth factor (NGF) potentiation
As mentioned above, the ZP-amides A–E (27–31) and qinbunamides A–C (34–36) were isolated from the pericarps of Z. bungeanum by Zhang and Gao in a study aimed at identifying natural products capable of promoting neurotrophic activity.35 As part of their research they assessed the ZP-amides 27, 29–31, and qinbunamides 35, and 36 (28 and 34 were omitted due to lack of material) for their ability to enhance NGF-mediated neurite outgrowth in rat pheochromo-cytoma (PC12) cells. Cytotoxicity studies confirmed that none of the alkamides investigated had any significant toxicity in PC12 cells or HCT116 human colon cancer cells at concentrations up to 50.0 μM. When combined with NGF, these six fatty acid amides (concentration of 20 μM) all exerted an increase in neurite-bearing cells compared to both negative control (DMSO) and positive control (NGF alone) cells. Diols 29 and 30, as well as ethyl ether 35, exhibited the strongest potentiation of NGF activity, with each consistently showing an approximately 4% boost in neurite bearing cells (p < 0.01 vs. NGF).4.5. Cancer
Several studies indicate that extracts for Zanthoxylum fruits, leaves, bark, and roots can exert noticeable anticancer activity.79 For example, extracts from Z. piperitum block the PAK1/cyclin D1 pathway as well as the growth of neurofibromatosis type 1 (NF1)-deficient cancer xenografts in mice.78,80 The majority of studies focused specifically on the potential of Zanthoxylum alkamides for the treatment of cancer, however, are limited to in vitro studies against various neoplastic cell lines. For example, several alkamides isolated from Nepalese Z. armatum were tested in a NF1- and p53-defective murine malignant glioma tumour cell line that compared loss of cell viability with growth inhibition in a dual-reporter assay (Table 4).33 Four of the ten Zanthoxylum alkamides tested demonstrated >50% growth inhibition, but only hydroxy-α-sanshool (2) did so without negatively impacting cell viability relative to untreated cells. The unique endo-peroxide timuramide A (38) also demonstrated favourable cell viability, albeit with relatively low concomitant growth inhibition activity.| Alkamide | Growth inhibition | Cell viabilitya |
|---|---|---|
| a Cell viability values are relative to untreated control cells in growth-retarding media using a red luminescent reporter (untreated cells = 100). Values less than 100 correspond to lowered cell viability relative to the untreated cells. | ||
| Hydroxy-α-sanshool (2) | 53% | 106 |
| Hydroxy-β-sanshool (5) | 65% | 56 |
| ZP-amide A (27) | 52% | 75 |
| ZP-amide C (29) | 31% | 79 |
| ZP-amide D (30) | 56% | 66 |
| ZP-amide E (31) | 41% | 67 |
| Timuramide A (38) | 38% | 97 |
| Timuramide B (39) | 13% | 65 |
| Timuramide C (40) | 47% | 66 |
| Timuramide D (41) | 42% | 70 |
Extracts from the fruit of Z. piperitum De Candolle inhibited cell proliferation and induced JNK-dependent autophagic cell death in three different human cancer cell lines (DLD-1, HepG2, and Caco-2).80 This is in line with previous studies which demonstrated that α-sanshool (1) induces apoptosis in human hepatocarcinoma HepG2 cells.81 It is possible that several different components of the fruit extract, in addition to 1 and other alkamides, were responsible for the observed pro-apoptotic activity. It should be noted that the anticancer activity of the Zanthoxylum fruit extract was not universal; A549, MCF-7 and WiDr cells, in addition to normal intestinal cells, were not affected.
Although these in vitro studies clearly demonstrate that certain Zanthoxylum alkamides exhibit noticeable activity against specific cancer cell lines, it is still premature to attribute genuine anticancer activity toward any of these compounds given that cancer is an in vivo phenomenon. Accordingly, more in vivo studies are required to verify if the observed in vitro results translate into a more physiologically relevant context.
4.6. Diabetes
Type-I and type-II diabetes are complicated and multi-faceted metabolic disorders caused by insulin resistance or the lack of insulin secretion. The transmembrane cannabinoid (CB) receptors play important roles in a wide variety of physiological processes, including glucose-mediated insulin secretion and T-cell activation. Of the three known CB receptors, CB1 and CB2 appear to be the most influential in regards to the progress of type-I diabetes. A current hypothesis is that blockade of the CB1 receptor with concomitant activation of the CB2 receptor might be a useful therapeutic option for the treatment of type-I diabetes. Several studies have revealed that structurally-similar alkamides isolated from various other plant genera (Echinacea,89–91Heliopsis,92Lepidium,92 and Otanthus93) exhibit extremely potent binding affinity for the CB2 and CB1 receptors, potentially explaining their associated anti-inflammatory activities. With this in mind, Dossou and co-worker screened a collection of alkamides extracted from Z. bungeanum pericarps with the hopes of identifying a dual CB1 antagonist/CB2 agonist.20 The initial CB1 and CB2 inhibition (antagonism) studies are summarized in Table 5. It should be noted that while the structure of δ-sanshool (10) was drawn, it was incorrectly referred to as γ-sanshool (7) in the original publication. Personal communication with the corresponding author (R. Moaddel) confirmed that the actual compound was 10 and not 7.† Both hydroxy-α-sanshool (2) and tetrahydrobungeanool (16) proved to be potent and selective CB2 antagonists, with the latter exhibiting greater potency and selectivity. Hydroxy-β-sanshool (5), on the other hand, was the most potent CB1 antagonist of the alkamides screened. Isobungeanool (17) and δ-sanshool (10) proved to be the weakest CB2 antagonists. Since the goal was to identify a dual CB1 antagonist/CB2 agonist, the extremely poor activity of 17 against CB1 precluded this compound from further investigation. The all-trans pentaene 10, on the other hand, was more promising. Further investigation confirmed that 10 was an effective CB2 agonist (EC50 of 41.7 nM), in addition to being an acceptable CB1 antagonist (IC50 of 100 nM). Accordingly, the authors proposed that alkamide 10 is a potential candidate for the treatment of type-I diabetes. From the current reports, a clear and concise structure–activity relationship as to what features in the alkamides make for a potent CB2 antagonist and/or CB1 agonist cannot be definitively established. Additionally, comparison to related alkamides isolated from non-Zanthoxylum sources are hampered by a lack of complete CB1/CB2 agonistic and antagonistic activity profiles. CB2 binding affinity studies for other alkamides, however, do suffer from similar inconclusive structure–activity relationships.89–93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20
| Alkamide | CB1 IC50 (nM) | CB2 IC50 (nM) | CB1/CB2 |
|---|---|---|---|
| Hydroxy-α-sanshool (2) | 7400 | 59 | 1254 |
| Dihydroxy-α-sanshool (3) | 41 | 168 | 0.24 |
| Hydroxy-β-sanshool (5) | 9 | 131 | 0.07 |
| Hydroxy-γ-sanshool (8) | 184 | 197 | 0.9 |
| δ-Sanshool (10) | 100 | 664 | 0.15 |
| Hydroxy-δ-sanshool (11) | 1392 | 224 | 6.2 |
| Tetrahydrobungeanool (16) | 4600 | 2 | 2556 |
| Isobungeanool (17) | 2400 | 9100 | 0.26 |
Very recent studies indicate that administration of Zanthoxylum-derived alkamides, specifically 2, 5, and 8, can ameliorate pancreatic dysfunction, protein metabolism disorder, and glycolipid metabolism in streptozotocin (SZT)-induced diabetic rats.82–84 These results lend further credence to the proposal that, despite their hydrophobic nature, Zanthoxylum-derived alkamides hold significant potential for the treatment of systemic (and not just topical) disorders.
4.7. Other activities
Several studies have been conducted on the antiparasitic activity of Zanthoxylum extracts, including the antileishmanial effect of extracts from Z. rhoifolium and potent activity against the malaria parasite exhibited by extracts from Z. heitzii.85,86 Although very little is currently known, early investigations indicate that natural products other than the alkamide components in these extracts are responsible for the observed antiparasitic activities.85 That being said, Navarrete and Hong identified α-sanshool (1) as the sole anthelmintic agent from a Z. liemannianum stem bark extract that was able to decrease the count of intestinal nematode eggs in naturally infected sheep.87 While low toxicity is generally associated with Zanthoxylum alkamides, the authors noted that intraperitoneal injection of 1 induced tonic–clonic seizures in mice.Among several other uses, Zanthoxylum decoctions have been employed worldwide in traditional medicines as anti-inflammatory agents. Despite this rich history, detailed clinical investigations on the anti-inflammatory capabilities of Zanthoxylum-derived alkamides are severely lacking. Last year, however, Wang and co-workers demonstrated that several alkamides isolated from Z. bungeanum could inhibit a process associated with inflammation, namely the production of nitric oxide (NO) in LPS-stimulated RAW264.7 cells.37 Of the alkamides tested, tetrahydrobungeanool (16, IC50 27.1 μM), unnamed diketone 33 (IC50 39.4 μM), zanthoamide A (42, IC50 48.7 μM), and ZP-amide A (27, IC50 49.8 μM) were the most potent inhibitors of NO production. It is worth noting that none of the twelve Zanthoxylum alkamides that were tested demonstrated any detectable cytotoxicity against the murine macrophages (up to 50.0 μM), nor against human colon cancer (HCT116) or human prostate cancer (PC-3) cells.
In their studies on the oral sensations exhibited by several isolated Zanthoxylum alkamides, Bader and co-workers also investigated the impact on salivation.18 Interestingly, the structural differences that evoked changes in oral sensation also expressed differences in the induction of salivation. For example, the cis-olefin-containing alkamide hydroxy-α-sanshool (2), which demonstrated tingling/paresthetic activity also induced massive salivation. The tongue-numbing all-trans congener hydroxy-β-sanshool (5), on the other hand, did not show any significant impact on saliva production.
There is a long history in the use of polyunsaturated fatty acid amides from various natural sources for several dermatological issues including eczema.94 Accordingly, it should come as no surprise that there is a commercial interest in the use of Zanthoxylum-alkamides for dermatological and cosmetic purposes. For example, a formulation comprised of a sanshool-rich extract from Z. bungeanum Maxim diluted with 80% oleyl alcohol (Zanthalene®)88 has been tested as an anti-itch cosmetic ingredient.95 Additionally, the lifting properties and anti-wrinkle effects of this formulation have been explored with noticeable but admittedly modest activity observed.96 Given the demonstrated activity of certain Zanthoxylum-derived alkamides with the cannabinoid receptors CB1 and CB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and the known beneficial effects of structurally-related alkamide CB2 ligands from other plant genera for dermatological disorders,94 Zanthalene® and similar formulations rich in Zanthoxylum alkamides hold significant potential in this arena.
20 and the known beneficial effects of structurally-related alkamide CB2 ligands from other plant genera for dermatological disorders,94 Zanthalene® and similar formulations rich in Zanthoxylum alkamides hold significant potential in this arena.
In addition to human health issues, the sanshools have recently gone under investigation for agricultural purposes. For example, Tang and co-workers reported that a collection of sanshools and hydroxy-sanshools (1–9, 12) extracted from Z. bungeanum pericarps was able to protect rice seedlings from the damaging effects of metolachlor treatment.23 Combination treatment of this sanshool mixture with the pesticide led to growth rates, shoot height, root length and fresh biomass close to that of non-treated controls, versus significantly lower values in all of these fields for seedlings treated with metolachlor alone. Accordingly, it is possible that Zanthoxylum-derived alkamides could serve as protective agents in combination with agricultural herbicides. It should also be noted that one of the earliest reported biological activities for α-sanshool (1) was its insecticidal properties (paralytic action and toxicity) against common house flies.12,97 In comparison to alkamides from other species, however, the insecticidal activity of the Zanthoxylum-derived polyunsaturated fatty acids remains relatively unexplored.7
5. Conclusions and perspectives
As discussed in this review, the polyunsaturated fatty acid amides produced by the Zanthoxylum genus of trees have intrigued and benefitted human societies throughout the world. With the advancement of science, these Zanthoxylum-derived alkamides have also advanced from simply culinary curiosities and components of folk remedies to legitimate clinical candidates and molecular probes for modern biology. Current studies adequately demonstrate that isomerization of the olefin stereochemistry in these alkamides (e.g.2 to 5) can dramatically alter both the degree and specific nature of the observed biological activities, thus emphasizing the importance of employing diastereomerically-pure compounds for all biological evaluations. While most studies rely on Zanthoxylum alkamides extracted and purified from natural sources, advances in organic synthesis now allow relatively rapid access to multi-gram quantities of some of the most intriguing members of this family of natural products as single polyolefin isomers. The Zanthoxylum alkamides still offer an attractive opportunity for further advances in organic synthesis and analysis. For example, five members of this family, namely lanyuamide IV (23), lanyuamide VI (25), ZP-amide F (32), timuramide A (38), and timuramide B (39), possess at least one chirality centre and optical activity. Nevertheless, the absolute configurations of these compounds remain unassigned. This is not without significant efforts. As mentioned in Section 2.6, Devkota and co-workers unsuccessfully attempted to determine the absolute stereochemistry of 38 by selectively reducing the endo-peroxide moiety and converting the resulting diol into the corresponding bis-Mosher's ester.33 The authors correctly note that such unsaturated diols represent a blind spot in current methods for configurational analysis. Accordingly, de novo total synthesis is the only strategy currently available to determine the absolute stereochemistry of the chiral Zanthoxylum alkamides. Whereas asymmetric syntheses of alcohols 23, 25, and 32 are certainly within reach using modern methods, access to the unique endo-peroxides 38 and 39 in enantiomerically-pure form from strictly synthetic means will require new methodological advances.Organic synthesis also has a lot to offer to the biomedical evaluation of Zanthoxylum alkamides. Current studies have focused primarily on the compounds that are easiest to isolate and purify from natural sources in significant quantities. Accordingly, the biological activities of the less prevalent members, such as the lanyuamides, qinbunamides, and zanthoamides, are not properly explored. Chemical synthesis is required to fill in this gap by providing access to larger quantities of these underrepresented alkamides. Moreover, the collection of synthetic analogues available for structure–activity relationship studies (see Section 3.6) is still relatively limited and lacking in compounds that recapitulate the desired biological activities without suffering the same sensitivities to light, heat, and hydroxylated solvents. Indeed, the discovery of a small molecule that demonstrates a similar activity profile to hydroxy-α-sanshool (2) against various ion channels but is more resilient would serve as a powerful molecular probe to elucidate the biomolecular mechanisms underlying the differences between pain, tickling, and itching. Similarly, the encouraging results observed with the ZP-amides and qinbunamides in potentiating NGF-induced neurite outgrowth necessitate a larger structure–activity relationship analysis using other Zanthoxylum alkamides and synthetic derivatives with the hopes of finding more active compounds and discovering the specific biomolecular target(s). One hope is that this review will spur interest in the synthetic organic chemistry community to channel some effort toward synthesizing these compounds and analogs thereof with the goal of gaining a better understanding of the various biological activities outlined herein.
6. Conflicts of interest
There are no conflicts to declare.7. Acknowledgements
J. J. C. received funding from the NSFC (Grant No. 21372159) and a visitorship grant from The University of Hong Kong. P. H. T. received funding from The University of Hong Kong.8. Notes and references
- S. Foster and J. A. Duke, A Field Guide to Medicinal Plants and Herbs of Eastern and Central North America The Peterson Field Guide Series, Houghton Mifflin Company, Boston, 2nd edn, 2000, pp. 268–269 Search PubMed.
- S. K. Adesina, Afr. J. Tradit., Complementary Altern. Med., 2005, 3, 282 Search PubMed.
- L. M. Perry, Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses, MIT Press, Cambridge, 1980, p. 370 Search PubMed.
- Z. Xie and X. Huang, Dictionary of Traditional Chinese Medicine, Commercial Press, Hong Kong, 1984, p. 175 Search PubMed.
- S. Y. Hu, Food Plants of China, Chinese University Press, Hong Kong, 2005, p. 504 Search PubMed.
- Y. Wang, Y. Ju and Z. Wang, Chin. Tradit. Herb. Drugs, 2002, 33, 666 CAS.
- H. Greger, Phytochem. Rev., 2016, 15, 729 CrossRef CAS.
- Y. Murayama and K. Shinozaki, J. Pharm. Soc. Jpn., 1931, 51, 379 CrossRef CAS.
- T. Aihara, J. Pharm. Soc. Jpn., 1950, 70, 405 CrossRef CAS.
- M. Jacobsen, J. Org. Chem., 1967, 32, 1646 CrossRef.
- L. Crombie, Nature, 1954, 174, 833 CrossRef CAS.
- L. Crombie, J. Chem. Soc., 1955, 995 RSC.
- L. Crombie and J. L. Tayler, J. Chem. Soc., 1957, 2760 RSC.
- I. Yasuda, K. Takeya and H. Itokawa, Chem. Pharm. Bull., 1981, 29, 1791 CrossRef CAS.
- I. Yasuda, K. Takeya and H. Itokawa, Phytochemistry, 1982, 21, 1295 CrossRef CAS.
- K. Mizutani, Y. Fukunaga, O. Taneka, N. Takasugi, Yl. Saruwatari, T. Fuwa, T. Yamauchi, J. Wang, M.-R. Jia, F.-Y. Li and Y.-K. Ling, Chem. Pharm. Bull., 1988, 36, 2362 CrossRef CAS.
- Y. Kashiwada, C. Ito, D. D. Kim, K. B. Oh, U. Oh and J. Shin, Arch. Pharmacal Res., 2008, 31, 569 CrossRef PubMed.
- M. Bader, T. D. Stark, C. Dawid, S. Lösch and T. Hofmann, J. Agric. Food Chem., 2014, 62, 2479 CrossRef CAS PubMed.
- Z.-F. Zhao, R.-X. Zhu, K. Zhong, Q. He, A.-M. Luo and H. Gao, J. Food Sci., 2013, 78, C1516–C1522 CrossRef CAS PubMed.
- K. S. S. Dossou, K. P. Devkota, C. Morton, J. M. Egan, G. Lu, J. A. Beutler and R. Moaddel, J. Nat. Prod., 2013, 76, 2060 CrossRef CAS PubMed.
- K. H. Jang, Y. H. Chang, D.-D. Kim, K.-B. Oh, U. Oh and J. Shin, Arch. Pharmacal Res., 2008, 31, 569 CrossRef CAS PubMed.
- K. S. S. Dossou, K. P. Devkota, P. V. Kavanagh, J. A. Beutler, J. M. Egan and R. Moaddel, Anal. Biochem., 2013, 437, 138 CrossRef CAS PubMed.
- X. Tang, X. Zhou, J. Wu, J. Li and L. Bai, Pestic. Biochem. Physiol., 2014, 110, 44 CrossRef CAS PubMed.
- Q. Xiong, D. Shi, H. Yamamoto and M. Mizuno, Phytochemistry, 1997, 46, 1123 CrossRef CAS.
- B. P. Bryant and I. Mezine, Brain Res., 1999, 842, 452 CrossRef CAS PubMed.
- Y. Kashiwada, C. Ito, H. Katagiri, I. Mase, K. Komatsu, T. Namba and Y. Ikeshiro, Phytochemistry, 1997, 44, 1125 CrossRef CAS.
- S. Huang, L. Zhao, X. L. Zhou, M. Ying, C. J. Wang and J. Wang, Chin. Chem. Lett., 2012, 23, 1247 CrossRef CAS.
- I.-S. Chen, T.-L. Chen, W.-Y. Lin, I.-L. Tsai and Y.-C. Chen, Phytochemistry, 1999, 52, 357 CrossRef CAS.
- I. L. Tsai, W. Y. Lin, M. W. Huang, T. L. Chen and I. S. Chen, Helv. Chim. Acta, 2001, 54, 779 Search PubMed.
- X. Yang, J. Agric. Food Chem., 2008, 56, 1689 CrossRef CAS PubMed.
- M. J. Cheng, I. L. Tsai and I. S. Chen, J. Chin. Chem. Soc., 2003, 50, 1241 CrossRef CAS.
- T. Hatano, K. Inada, T.-o. Ogawa, H. Ito and T. Yoshida, Phytochemistry, 2004, 65, 2599 CrossRef CAS PubMed.
- K. P. Devkota, J. Wilson, C. J. Henrich, J. B. McMahon, K. M. Reilly and J. A. Beutler, J. Nat. Prod., 2013, 76, 59 CrossRef CAS PubMed.
- S. Y. Yang, B. H. Tai, S. B. Song, W. Li, X. T. Yan, Y. N. Sun, N. P. Thao and Y. H. Kim, Bull. Korean Chem. Soc., 2014, 35, 2361 CrossRef CAS.
- J.-M. Tian, Y. Wang, Y.-Z. Xu, Z.-C. Yu, A.-Z. Wei, W.-M. Zhang and J.-M. Gao, Bioorg. Med. Chem. Lett., 2016, 26, 338 CrossRef CAS PubMed.
- T. Mae, S. Yokota, M. Kuroda, Y. Mimaki and Y. Sashida, WO2005048737A1, 2005.
- Y. Wang, C.-H. Li, B. Luo, Y. N. Sun, Y. H. Kim, A.-Z. Wei and J.-M. Gao, Molecules, 2016, 21, 1416 CrossRef PubMed.
- J. M. Gulland and G. U. Hopton, J. Chem. Soc., 1930, 6, 6–11 RSC.
- S. K. Adesina and D. D. Akinwusi, J. High Resolut. Chromatogr., 1986, 9, 412 CAS.
- S. K. Adesina, J. Nat. Prod., 1986, 49, 715 CrossRef CAS.
- Y. K. Askoua, C. C. Philomene, H. Ramiarantsoa, E. Prost, D. Harakat, E. Le Magrex-Debar, S. C. Gangloff, A. Ahibo Coffy and M. Zeches-Hanrot, C. R. Chim., 2015, 18, 891 CrossRef.
- N. Moussavi, K. E. Malterud, B. Mikolo, D. Dawes, F. Chandre, V. Corbel, D. Massamba, H. J. Overgaard and H. Wangensteen, Parasites Vectors, 2015, 8, 503 CrossRef PubMed.
- H. Wangensteen, G. Ho, T. Thanh, M. Tadesse, C. O. Miles, N. Moussavi, B. Mikolo and K. E. Materud, Fitoterapia, 2016, 109, 196 CrossRef CAS PubMed.
- H. Shibuya, Y. Takeda, R.-s. Zhang, R.-X. Tong and I. Kitagawa, Chem. Pharm. Bull., 1992, 40, 2325 CrossRef CAS PubMed.
- P. E. Sonnet, J. Org. Chem., 1969, 34, 1147 CrossRef CAS.
- L. Crombie and D. Fisher, Tetrahedron Lett., 1985, 26, 2477 CrossRef CAS.
- L. Crombie and D. Fisher, Tetrahedron Lett., 1985, 26, 2481 CrossRef CAS.
- B. Wu, L. Kun and P. H. Toy, Synlett, 2012, 23, 2564 CrossRef CAS.
- X. Xia and P. H. Toy, Synlett, 2014, 25, 2787 CrossRef CAS.
- Y. Igarashi, K. Aoki, H. Nishimura, I. Morishita and K. Usui, Chem. Pharm. Bull., 2012, 60, 1088 CrossRef CAS PubMed.
- K. Aoki, Y. Igarashi, H. Nishimura, I. Morishita and K. Usui, Tetrahedron Lett., 2012, 53, 6000 CrossRef CAS.
- C. Mugnaini and F. Corelli, Synthesis, 2016, 48, 2085 CrossRef CAS.
- C. C. Galopin, S. M. Furrer and A. Goeke, Pungent and Tingling Compounds in Asian Cuisin, in Challenges in Taste Chemistry and Biology, ed. T. Hofman, C. T. Ho and W. Pickenhagen, ACS Symposium Series 876, American Chemical Society, Washington, DC, USA, 2004, pp. 139–152 Search PubMed.
- C. Menozzi-Smarrito, C. E. Riera, C. Munari, J. Le Coutre and F. Robert, J. Agric. Food Chem., 2009, 57, 1982 CrossRef CAS PubMed.
- R.-X. Zhu, K. Zhong, W.-C. Zeng, X.-Y. He, X.-Q. Gu, Z.-F. Zhao and H. Gao, Afr. J. Microbiol. Res., 2011, 5, 4631 CAS.
- R. Rong, M.-Y. Cui, Q.-L. Zhang, M.-Y. Zhang, Y.-M. Yu, X.-Y. Zhou, Z.-G. Yu and Y.-L. Zhao, J. Sep. Sci., 2016, 39, 2728 CrossRef CAS PubMed.
- M. Munekage, H. Kitagawa, K. Ichikawa, J. Watanabe, K. Aoki, T. Kono and K. Hanazaki, Drug Metab. Dispos., 2011, 39, 1784 CrossRef PubMed.
- M. Munekage, K. Ichikawa, H. Kitagawa, K. Ishihara, H. Uehara, J. Watanabe, T. Kono and K. Hanazaki, Drug Metabol. Dispos., 2013, 41, 1256 CrossRef CAS PubMed.
- E. Sugai, Y. Morimitsu, Y. Iwasaki, A. Morita, T. Watanabe and K. Kubota, Biosci. Biotechnol. Biochem., 2005, 69, 1951 CrossRef CAS PubMed.
- C. Dawid, A. Henze, O. Frank, A. Glabasnia, M. Rupp, K. Büning, D. Orlikowski, M. Bader and T. Hofmann, J. Agric. Food Chem., 2012, 60, 2884 CrossRef CAS PubMed.
- J. Y. Koo, Y. Jang, H. Cho, C.-H. Lee, K. H. Jang, Y. H. Chang, J. Shin and U. Oh, Eur. J. Neurosci., 2007, 26, 1139 CrossRef PubMed.
- C. E. Riera, C. Menozzi-Smarrito, M. Affolter, S. Michlig, C. Munari, F. Robert, H. Vogel, S. A. Simon and J. le Coutre, Br. J. Pharmacol., 2009, 157, 1398 CrossRef CAS PubMed.
- D. M. Bautista, Y. M. Sigal, A. D. Milstein, J. L. Garrison, J. A. Zorn, P. R. Tsuruda, R. A. Nicoll and D. Julius, Nat. Neurosci., 2008, 11, 772 CrossRef CAS PubMed.
- R. C. Lennertz, M. Tsunozaki, D. M. Bautista and C. L. Stucky, J. Neurosci., 2010, 30, 4353 CrossRef CAS PubMed.
- M. Tsunozaki, R. C. Lennertz, D. Vilceanu, S. Katta, C. L. Stucky and D. M. Bautista, J. Physiol., 2013, 591, 3325 CrossRef CAS PubMed.
- N. Hagura, H. Barber and P. Haggard, Proc. R. Soc. B, 2013, 280, 20131680 CrossRef PubMed.
- S. Kuroki, N. Hagura, S. Nishida, P. Haggard and J. Watanabe, PLoS One, 2016, 11, e0165842 Search PubMed.
- T. Kono, M. Shimada, M. Yamamoto, A. Kaneko, Y. Oomiya, K. Kubota, Y. Kase, K. Lee and Y. Uezono, Front. Pharmacol., 2015, 6, 159 CrossRef PubMed.
- X.-L. Jin, C. Shibata, H. Naito, T. Ueno, Y. Funayama, K. Fukushima, S. Matsuno and I. Sasaki, Dig. Dis. Sci., 2001, 46, 1171 CrossRef CAS PubMed.
- N. Kawasaki, K. Nakada, T. Nakayoshi, Y. Furukawa, Y. Suzuki, N. Hanyu and K. Yanaga, Dig. Dis. Sci., 2007, 52, 2684 CrossRef PubMed.
- T. Kono, T. Kanematsu and M. Kitajima, Surgery, 2009, 146, 837 CrossRef PubMed.
- T. Itoh, J. Yamakawa, M. Mai, N. Yamaguchi and T. Kanda, J. Int. Med. Res., 2002, 30, 428 CrossRef CAS PubMed.
- Y. Tokita, K. Satoh, M. Sakaguchi, Y. Endoh, I. Mori, M. Yuzurihara, I. Sakakibara, Y. Kase, S. Takeda and H. Sasaki, Inflammopharmacology, 2007, 15, 65 CrossRef CAS PubMed.
- Y. Tokita, M. Yuzurihara, M. Sakaguchi, K. Satoh and Y. Kase, J. Pharmacol. Sci., 2007, 104, 303 CrossRef CAS.
- K. Kubota, N. Ohtake, K. Ohbuchi, A. Mase, S. Imamura, Y. Sudo, K. Miyano, M. Yamamoto, T. Kono and Y. Uezono, Am. J. Physiol. Gastrointest. Liver Physiol., 2015, 308, G579 CrossRef CAS PubMed.
- Y. Tokita, H. Akiho, K. Nakamura, E. Ihara and M. Yamamoto, J. Pharmacol. Sci., 2015, 127, 344 CrossRef CAS PubMed.
- K. Tsuchiya, K. Kubota, K. Ohbuchi, A. Kaneko, N. Ohno, A. Mase, H. Matsushima, M. Yamamoto, K. Miyano, Y. Uezono and T. Kono, Neurogastroenterol. Motil., 2016, 28, 1792 CrossRef CAS PubMed.
- Y. Hirokawa, T. Nheu, K. Grimm, V. Mautner, S. Maeda, M. Yoshida, K. Komiyama and H. Maruta, Cancer Biol. Ther., 2006, 5, 305 CrossRef PubMed.
- Y. Yang, T. Ikezoe, T. Takeuchi, Y. Adachi, Y. Ohtsuki, H. P. Koeffler and H. Taguchi, Oncol. Rep., 2006, 15, 1581 Search PubMed.
- R. Nozaki, T. Kono, H. Bochimoto, T. Watanabe, K. Oketani, Y. Sakamaki, N. Okubo, K. Nakagawa and H. Takeda, Oncotarget, 2016, 7, 70437 Search PubMed.
- Y. You, M. Zhou, H. Lu, G. G. Shirima, Y. Cheng and X. Liu, Food Sci. Biotechnol., 2015, 24, 2169 CrossRef CAS.
- Y. You, T. Ren, S. Zhang, G. G. Shirima, Y.-J. Cheng and X. Liu, Food Funct., 2015, 6, 3144 CAS.
- T. Ren, Y. Zhu and J. Kan, Clin. Exp. Hypertens., 2017, 39, 330 CrossRef CAS PubMed.
- T. Ren, Y. Zhu, X. Xia, Y. Ding, J. Guo and J. Kan, J. Mol. Endocrinol., 2017, 58, 113 CrossRef CAS PubMed.
- C. D. Goodman, I. Austarheim, V. Mollard, B. Mikolo, K. E. Malterud, G. I. McFadden and H. Wangensteen, Malar. J., 2016, 15, 481 CrossRef PubMed.
- B. M. Neto, J. M. S. R. Leitao, L. G. C. Oliveira, S. E. M. Santos, S. M. P. Carneiro, K. A. F. Rodrigues, M. H. Chaves, D. D. R. Arcanjo and F. A. A. Carvalho, An. Acad. Bras. Cienc., 2016, 88(suppl. 3), 1851 CrossRef PubMed.
- A. Navarrete and E. Hong, Planta Med., 1996, 62, 250 CrossRef CAS PubMed.
- E. Bombardelli and B. Gabetta, WO00/02570, Jan 20, 2000.
- J. Hohmann, D. Rédei, P. Forgo, P. Szabó, T. F. Freund, J. Haller, E. Bojnik and S. Benyhe, Phytochemicals, 2011, 72, 1848 CrossRef CAS PubMed.
- J. Gertsch, S. Raduner and K. H. Altmann, J. Recept. Signal Transduction Res., 2006, 26, 709 CrossRef CAS PubMed.
- S. Raduner, A. Majewska, J. Z. Chen, X. Q. Xie, J. Hamon, B. Faller, K. H. Altmann and J. Gertsch, J. Biol. Chem., 2006, 281, 14192 CrossRef CAS PubMed.
- Z. Hajdu, S. Nicolussi, M. Rau, L. Lorántfy, P. Forgo, J. Hohmann, D. Csupor and J. Gertsch, J. Nat. Prod., 2014, 77, 1663 CrossRef CAS PubMed.
- S. Ruiu, N. Anzani, A. Orrù, C. Floris, P. Caboni, E. Maccioni, S. Distinto, S. Alcaro and F. Cottiglia, Bioorg. Med. Chem., 2013, 21, 7074 CrossRef CAS PubMed.
- A. Oláh, J. Szabó-Papp, M. Soeberdt, U. Knie, S. Dähnhardt-Pfeiffer, C. Abels and T. Bíró, J. Dermatol. Sci., 2017, 88, 67 CrossRef PubMed.
- G. Guglielmini and A. Cristoni, Cosmet. Toiletries, 2002, 7, 47 Search PubMed.
- C. Artaria, G. Maramaldi, A. Bonfigli, L. Rigano and G. Appendino, Int. J. Cosmet. Sci., 2011, 33, 328 CrossRef CAS PubMed.
- F. B. LaForge, H. L. Haller and W. N. Sullivan, J. Am. Chem. Soc., 1942, 64, 187 CrossRef CAS.
Footnote |
| † Private communication between P. H. Toy and R. Moaddel (NIH). |
| This journal is © The Royal Society of Chemistry 2018 |