Recent progress in the isolation, bioactivity, biosynthesis, and total synthesis of natural spiroketals
Fu-Min
Zhang
 a,
Shu-Yu
Zhang
a,
Shu-Yu
Zhang
 b and
Yong-Qiang
Tu
b and
Yong-Qiang
Tu
 *ab
*ab
aState Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China
bSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: tuyq@lzu.edu.cn; tuyq@sjtu.edu.cn
First published on 22nd January 2018
Abstract
Covering: 2011 to July 2017.
Spiroketal (spiroacetal), a common moiety in numerous natural products, drugs and functional molecules, has been a central topic in organic chemistry for a long time. Owing to their structural diversity, important bioactivity and functional irreplaceability, natural spiroketals have attracted the interest of natural product chemists, medical chemists, biological chemists, agricultural chemists, synthetic chemists, and chemical biologists. In this review, we focus on the overview of the isolation, bioactivity, biosynthesis and total synthesis of spiroketals from 2011 to July 2017.
1. Introduction
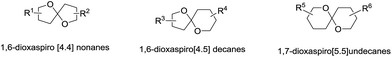
Spiroketal, also called spiroacetal, is a unique moiety that occurs in numerous natural products, drugs and functional molecules. Structurally, the spiroketal contains at least two oxacyclic rings, in which the oxygen atoms belonging to different rings share a common spiro-carbon atom. Spiroketal is designated as a substituted spirane base in systematic nomenclature or as an [m,n]-spiroketal in customary nomenclature. There are three common ring systems, 1,6-dioxaspiro[4.4]nonane (also [5,5]-spiroketal), 1,6-dioxaspiro[4.5]decane (also [5,6]-spiroketal), and 1,7-dioxaspiro[5.5]undecane (also [6,6]-spiroketal), while other ring systems are relatively seldom observed (Fig. 1).Because of the inherent spiro-chiral centre in spiroketals, their diastereoisomers and enantiomers have special terms, and these issues have been extensively discussed in previously published reviews and books.1–6 In particular, the anomeric effect in the spiroketal system plays a critical role in its conformational analysis, its bioactive contributions and the design of total synthesis strategies. Generally, natural spiroketals have a stabilizing anomeric effect; however, exceptions have also been found in some spiroketal scaffolds due to the presence of substituents or an intramolecular H-bond.7
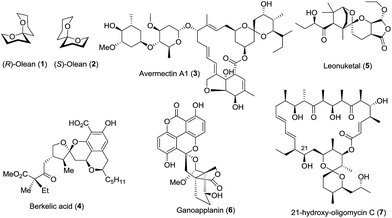
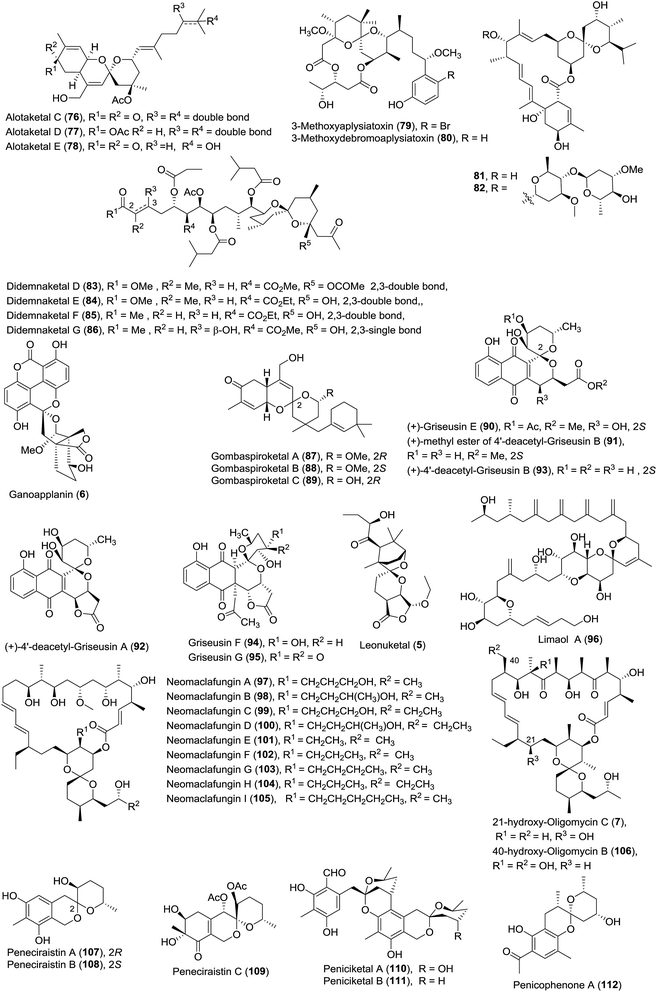
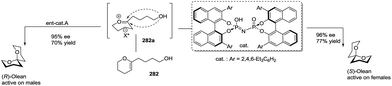
Due to their diverse ring structures and varied substituents, spiroketals exhibit wide and various bioactivities. For example, (R)-olean (1) is active in males, while (S)-olean (2) is active in females;8 avermectin A1 (3), along with its derivatives, is an important pesticide for the treatment of parasitic worms.9 Berkelic acid (4) shows broad bioactivities including the selective inhibition of OVCAR-3 (GI50 = 91 nM) and the pronounced inhibition of the matrix metalloproteinase MMP3 (GI50 = 1.8 μM).10 Leonuketal (5) exhibits vasorelaxant activity with EC50 = 2.32 μM,11 and ganoapplanin (6) displays inhibitory activity against T-type voltage-gated calcium channels.12 Interestingly, 21-hydroxy-oligomycin C (7) has proved to be an exceptionally potent inhibitor of K-Ras PM localization with an IC50 value of approximately 6 nM (Fig. 2).13
Although new chemical structures, the investigation of their corresponding activity, biosynthesis, and total synthesis of spiroketals have been reported in many articles and some reviews,14–16 no systematic collection and classification has yet been performed. In this review, we have systematically summarized the isolation, bioactivities and possible biosynthetic pathways of new natural spiroketals reported from 2011 to July 2017, and have highlighted recent representative examples of the biosynthesis and total synthesis of natural spiroketals during the same period.
The abovementioned spiroketal descriptions imply that each ring contains only one oxygen atom. Spiroketals having more than one oxygen atom17 or involving another heteroatom18 are omitted from this review.
Spiroketal skeletons also occur embedded in other structural moieties, including alkaloids,19 and steroids.20 Although these embedded spiroketals are structurally important units, they are also not included in this review.
2. Isolation, bioactivities and biosynthesis
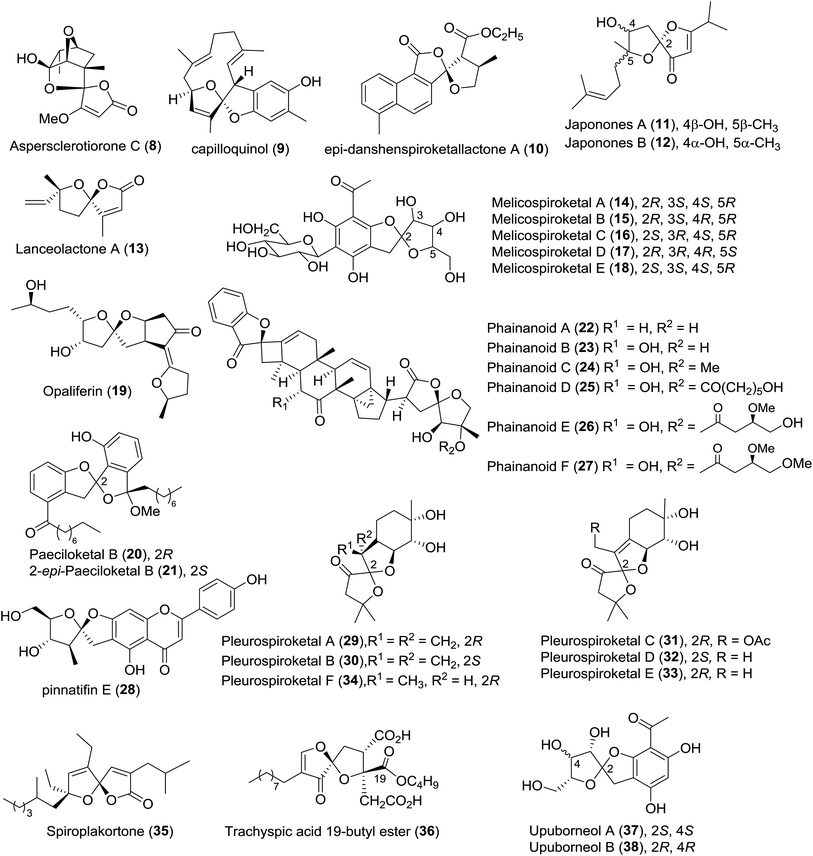
The isolation of new spiroketals and the investigation of biological functions are still a growing field of natural product chemistry. In the past seven years, more than one hundred new spiroketals with unusual skeletons have been isolated from various sources including plants, animals, and microbes. In this section, we provide an overview of the isolation and bioactivities of “new” spiroketals according to a classification based on the size of the spiroketal ring, such as [5,5]-, [5,6]-, [6,6]-, and [5,7]-spiroketals. These spiroketals with different ring systems are tabulated below, and the tables include their names, source organisms, structural features, biological activities, and related references; they are then described individually according their chemical structures. Finally, the biosynthesis of some spiroketals is also discussed.21,222.1 [5,5]-Spiroketals
The newly isolated [5,5]-spiroketals are listed in Table 1, and their chemical structures are shown in Fig. 3.| Entries | Compound names/number | Isolation source | Structural elucidation | Structural classification | Bioactivity | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Aspersclerotiorone C (8) | Fungus Aspergillus sclerotiorum PSU-RSPG178 | HRMS (ESI), IR, UV, 1D- and 2D-NMR, CD | Spirofuranone-γ-butenolide | — | 23 | |
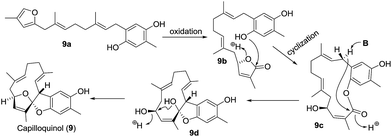
| 2 | Capilloquinol (9) | Dongsha Atoll soft coral Sinularia capillosa | HRMS (ESI), IR, 1D- and 2D-NMR | Farnesyl quinoid | Cytotoxicity; antiviral activity | 24 | |
| 3 | epi-Danshenspiroketallactone A (10) | Cell cultures of Salvia miltiorrhiza | HRMS (ESI), IR, 1D- and 2D-NMR, CD | Diterpenoids | Low antitumor activity | 25 | |
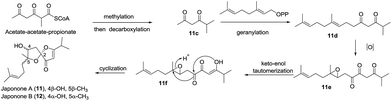
| 4 | Japonones A–B (11–12) | Hypericum japonicum Thunb. | HRMS (ESI), UV, IR, 1D- and 2D-NMR, Mosher's method, ECD | Anti-KSHV activity; antiviral activity | 26 | ||
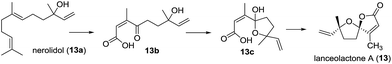
| 5 | Lanceolactone A (13) | Leaves of Illicium lanceolatum | HRMS (CI), IR, 1D- and 2D-NMR, CD | Tetranorsesquiterpenoid | Antimicrobial activity | 27 | |
| 6 | Melicospiroketals A–E (14–18) | Leaves of Melicope pteleifolia | HRMS (ESI), IR, 1D- and 2D-NMR | Polyphenol | — | 28 | |
| 7 | Opaliferin (19) | Insect pathogenic fungus Cordyceps sp. NBRC 106954 | HRMS (EI), 1D- and 2D-NMR, XRD | Polyketide | Weak cytotoxicity | 29 | |
| 8 | Paeciloketal B (20) and 2-epi-paeciloketal B (21) | Derived fungus Paecilomyces variotii J08NF-1 | FAB-CIDMS/MS, HRMS (FAB), 1D- and 2D-NMR, ECD | Benzannulated spiroketal | — | 30 | |
| 9 | Phainanoids A–F (22–27) | Phyllanthus hainanensis | HRMS (ESI), IR, 1D- and 2D-NMR, Cu Kα XRD, CD | Triterpenoids | Immunosuppressive activity against proliferation of T and B lymphocytes | 31 | |
| 10 | Pinnatifin E (28) | Seeds of Vaccaria segetalis | HRMS (TOF), IR, 1D- and 2D-NMR | Flavonoidal glycoside | Weak inhibitory activity against factor Xa | 32 | |
| 11 | Pleurospiroketals A–F (29–34) | Edible mushroom Pleurotus cornucopiae | HRMS (ESI), 1D- and 2D-NMR, Cu Kα XRD, Mosher's method, CD | Sesquiterpene | Inhibitory activity against nitric oxide production | 33 and 34 | |
| 12 | Spiroplakortone (35) | Chinese sponge Plakortis simplex | HRMS (ESI), IR, 1D- and 2D-NMR, MMFF conformational analysis, DP4 TDDFT calculation, ECD | Polyketide | Moderate cytotoxicity | 35 | |
| 13 | Trachyspic acid 19-butyl ester (36) | Uncharacterized fungus RKGS-F2684 | NPPlot, IR, UV, HRMS (FAB), 1D- and 2D-NMR | Spiroacetal | Inhibitor of PBD-dependent binding (IC50 = 102 μM) | 36 | |
| 14 | Upuborneols A and B (37–38) | Leaves of Upuna borneensis Sym. | MS (ESI), IR, 1D- and 2D-NMR | Acetophenone | — | 37 |
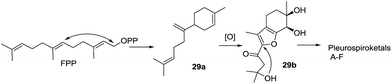
Pleurospiroketals A–F (29–34) feature novel perhydrobenzannulated sesquiterpenes, and the position of the double bond and the absolute configuration of the spiroatom are the major differences.33,34 The assay of bioactivity against nitric oxide (NO) production in the lipopolysaccharide-activated macrophage cell RAW 264.7 revealed that pleurospiroketals A–C showed NO inhibitory activity with IC50 values of 6.8, 12.6, and 20.8 μM, respectively, and the preliminary structure–activity relationship analysis proved that the exocyclic double bond was beneficial for NO inhibitory activity. Additionally, pleurospiroketals A–C exhibited cytotoxicity against the HeLa cell line with IC50 values of 20.6, 32.8, and 18.8 μM, respectively. Compound 29a, derived from FPP, was proposed as the possible biosynthetic precursor of pleurospiroketals A–F (Scheme 2). It can be subsequently oxidized to introduce multiple oxygen-containing groups and to construct the multi-substituted furan 29b, then further transformed into pleurospiroketals A–F.
An assay of cytotoxic activities of epi-danshenspiroketallactone A (10) against five human cell lines (HCT-8, Bel-7402, BGC-823, A549, and A2780) revealed no significant activity.25 In the ongoing search for immunosuppressive agents from Chinese medicinal herbs, Yue and coworkers isolated phainanoids A–F (22–27), six structurally novel triterpenoids incorporating a unique [5,5]-spirocyclic system in 2015.31 Notably, phainanoid F (27) exhibited remarkable activities against the proliferation of T-cells (IC50 = 2.04 ± 0.01 nM) and B-cells (IC50 < 1.60 ± 0.01 nM). The unique structure and interesting immunosuppressive bioactivity of these spiroketals open a new window for the exploration of new lead compounds.
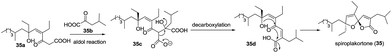
Spiroplakortone (35) features an unprecedented γ-spiroketal-γ-lactone moiety and it displays moderate cytotoxicity against the L5178Y cell line (IC50 = 37.5 μM).35 A plausible biosynthetic pathway of spiroplakortone was postulated (Scheme 4). An aldol reaction between β-ketoacid (35a) and α-ketoisocaproate (35b) derived from the biotransformation of leucine gave the adduct 35c, which was decarboxylated and then cyclized to afford spiroplakortone.
In 2015, Jung and colleagues described the isolation and identification of paeciloketal B (20) and 2-epi-paeciloketal B (21), which share a rare benzannulated [5,5]-spiroketal skeleton.30 The combination of the high-throughput screening system for polo box domain (PBD) inhibitors and the methodology of the fractionation of microbial metabolites with a spectral database is an alternative tool in the search for new natural products, and using this method, Osada and colleagues isolated a new 5,5-spiroacetal metabolite, trachyspic acid 19-butyl ester (36).36 Although this compound may be an artefact derived from trachyspic acid due to using butanol in the extraction process, it should be an attractive lead compound for polo-like kinase 1 (PlK1) inhibition.
2.2 [5,6]-Spiroketals
The newly isolated [5,6]-spiroketals are listed in Table 2, and their chemical structures are shown in Fig. 4 and 5.| Entries | Compound names/number | Isolation source | Structural elucidation | Structural classification | Bioactivity | Ref. |
|---|---|---|---|---|---|---|
| 1 | Acuminolide A (39) | The dinoflagellate Dinophysis acuminata | HRMS (ESI), IR, UV, 1D- and 2D-NMR | Macrolide | Stimulator of actomyosin ATPase | 38 |
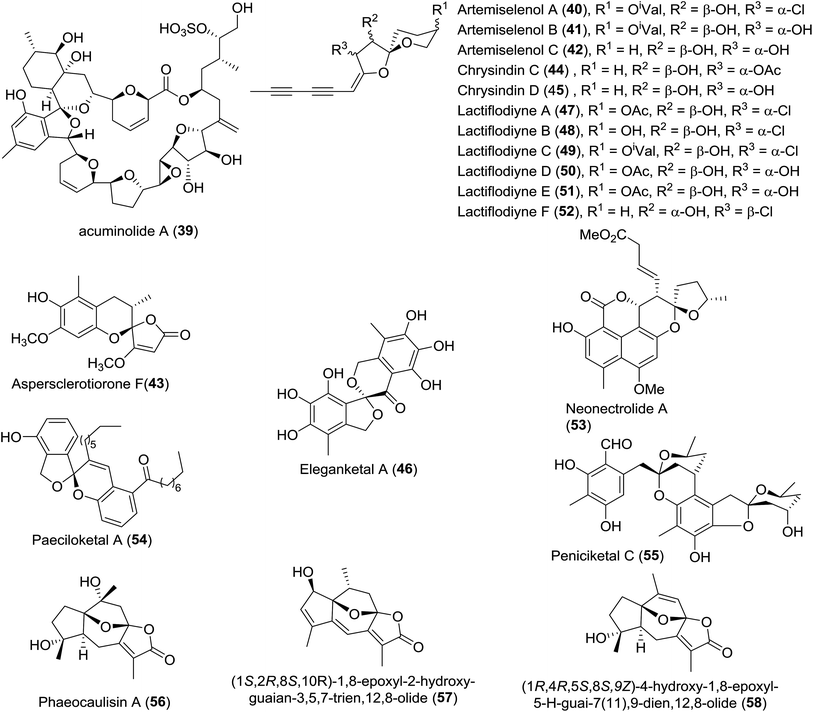
| 2 | Artemiselenols A–C (40–42) | Artemisia selengensis Turcz. (Compositae) | HRMS (ESI), UV, 1D- and 2D-NMR, XRD, CD | — | Neuroprotection activity; the inhibition of human monoamine oxidase (hMAOs) in vitro | 39 |
| 3 | Aspersclerotiorone F (43) | Fungus Aspergillus sclerotiorum PSU-RSPG178 | HRMS (ESI), UV, IR, 1D- and 2D-NMR, CD | γ-Butenolide/furanone | — | 23 |
| 4 | Chrysindins C–D (44–45) | The flowers of Chrysanthemum indicum | HRMS (ESI), IR, 1D- and 2D-NMR, CD | Polyacetylene | — | 40 |
| 5 | Eleganketal A (46) | Fungus Spicaria elegans KLA03 | HRMS (ESI), UV, 1D- and 2D-NMR, CD, TDDFT-ECD, synthesis of its analogue | Polyketide | — | 41 |
| 6 | Lactiflodiynes A–F (47–52) | Artemisia lactiflora | HRMS (EI), IR, UV, 1D- and 2D-NMR, XRD, CD, chemical transformation | Diacetylenic spiroacetal enol ethers | — | 42 |
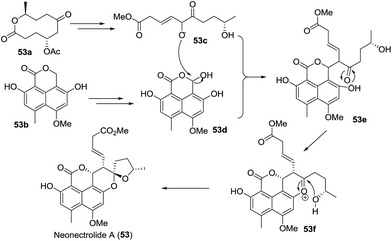
| 7 | Neonectrolide A(53) | Fungus Neonectria sp. | HRMS (ESI), 1D- and 2D-NMR, ECD, CD, TDDFT | Oxaphenalenone | Cytotoxicity | 43 |
| 8 | Paeciloketal A (54) | Jellyfish-derived fungus Paecilomyces variotii J08NF-1 | MS (FAB-CID), HRMS (FAB), 1D- and 2D-NMR, ECD, TDDFT | Octaketide | Antibacterial activity | 30 |
| 9 | Peniciketal C (55) | Saline soil derived Penicillium raistrichii | HRMS (ESI), 1D- and 2D-NMR, CD, ECD | — | Cytotoxicity | 44 |
| 10 | Phaeocaulisin A and analogues (56–58) | Rhizomes of Curcuma phaeocaulis; gorgonian Menella woodin; Curcuma kwangsiensis | HRMS (ESI), IR, UV, 1D- and 2D-NMR, XRD, CD | Sesquiterpenes | Inhibitory activities against NO production | 45–47 |
| 11 | Phosphocalyculin C (59) | The marine sponge Discodermia calyx | HRMS (FAB), 1H NMR, 13C NMR, 31P, LC-MS | Pyrophosphate derivative | Cytotoxicity | 48 |
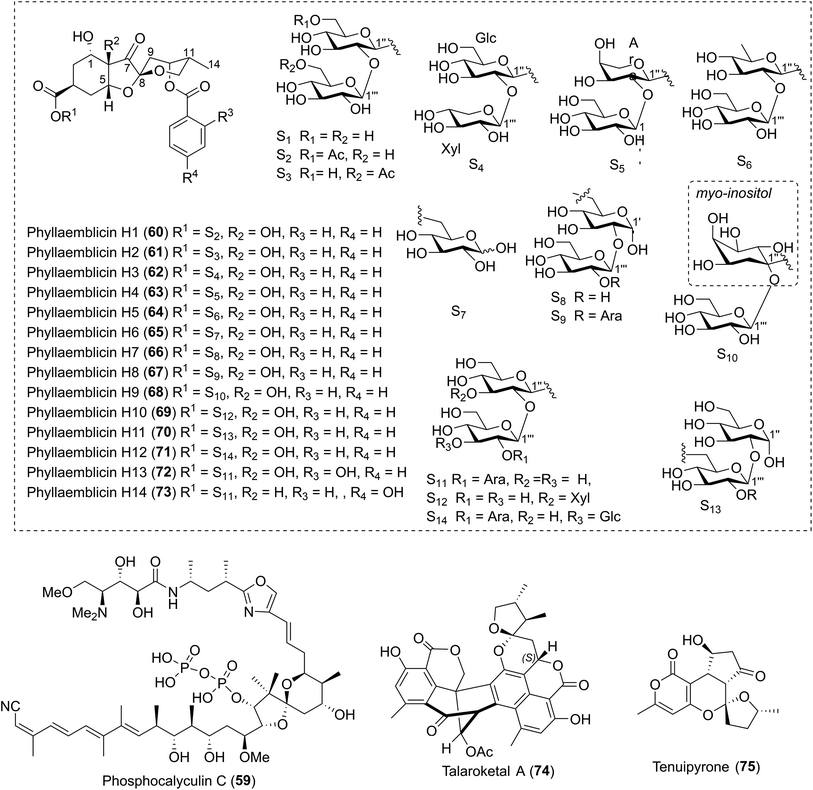
| 12 | Phyllaemblicins H1–H14 (60–73) | The roots of Phyllanthus emblica Linn. | HRMS (ESI), IR, UV, 1D- and 2D-NMR, ECD | Sesquiterpenoid | Moderate cytotoxicity; HSV-1 inhibitory activity; anti-CVB3 cell activities | 49 |
| 13 | Talaroketal A (74) | The soil fungus Talaromyces stipitatus ATCC 10500 | HRMS (ESI), IR, 1D- and 2D-NMR, XRD, ECD | Oxaphenalenone dimer | Antimicrobial activity against Staphylococcus aureus | 50 |
| 14 | Tenuipyrone (75) | The entomopathogenic fungus Isaria tenuipes | HRMS (EI), UV, IR, 1D- and 2D-NMR, CD, XRD | Polyketide | — | 51 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 and 58,47 were also identified, respectively. Unfortunately, no related bioactivities were reported. Fourteen highly oxygenated norbisabolane sesquiterpenoid-type spiroketals, phyllaemblicins H1–H14 (60–73), were isolated by Zhang and coworkers.49 The major structural differences lay in the different sugar substituents. Phyllaemblicin H1 (60) exhibited moderate cytotoxicity against the human cancer cell lines A-549 (IC50 = 4.7 ± 0.7 μM) and SMMC-7721 (IC50 = 9.9 ± 1.3 μM). Phyllaemblicin H6 (65) displayed inhibitory activity against HSV-1, whereas phyllaemblicin H8 (67) and phyllaemblicin H12 (71) showed moderate anti-CVB3 cell activities.
46 and 58,47 were also identified, respectively. Unfortunately, no related bioactivities were reported. Fourteen highly oxygenated norbisabolane sesquiterpenoid-type spiroketals, phyllaemblicins H1–H14 (60–73), were isolated by Zhang and coworkers.49 The major structural differences lay in the different sugar substituents. Phyllaemblicin H1 (60) exhibited moderate cytotoxicity against the human cancer cell lines A-549 (IC50 = 4.7 ± 0.7 μM) and SMMC-7721 (IC50 = 9.9 ± 1.3 μM). Phyllaemblicin H6 (65) displayed inhibitory activity against HSV-1, whereas phyllaemblicin H8 (67) and phyllaemblicin H12 (71) showed moderate anti-CVB3 cell activities.
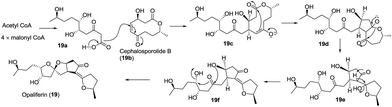
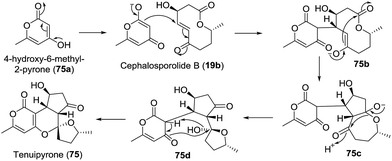
Screening of the secondary metabolites produced by entomopathogenic fungi grown in the presence of epigenetic modifying agents is a possible method to find new secondary metabolite products. Using the abovementioned approach, Oshima isolated a novel spiroketal bearing an unprecedented tetracyclic ring system, named tenuipyrone (75).51 A plausible biosynthetic route is postulated (Scheme 7). Michael addition between 4-hydroxy-6-methyl-2-pyrone (75a) and cephalosporolide B (19b) would result in the enolated intermediate 75b. Claisen condensation, rearrangement, and spiroketalization would give tenuipyrone.
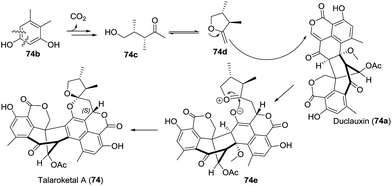
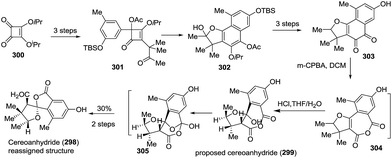
Very recently, Prado and coworkers isolated talaroketal A (74), a novel oxaphenalenone dimer.50 It features a rare benzannulated [5,6]-spiroketal and the dimeric bis(oxaphenalenone) skeleton, and it displayed modest antimicrobial activity against Staphylococcus aureus. The biosynthetic precursor of talaroketal A was proposed based on the co-isolation of duclauxin (74a), which possesses the same skeleton except for the spiroketal unit, and the substituted exo-enol (74d), derived from compound 74b, was proposed as another biosynthetic partner. The reaction of duclauxin 74a with enol 74d yielded the intermediate 74e, which was subsequently spiro-cyclized to produce talaroketal A (Scheme 9).
Structurally, eleganketal A (46) is a rare naturally occurring spiroketal bearing a spiro[isobenzofuran-1,3′-isochroman] ring system.41
In 2015, paeciloketal A (54), a new benzannulated [5,6]-spiroketal bearing a sole chiral spiro-stereocentre, was isolated as a mixture of two enantiomers by Jung and colleagues.30 Interestingly, the authors found that a pair of enantiomers of paeciloketal A could interconvert when they were dissolved in methanol at room temperature, which indicated that the ketal formation was reversed. This case is a rare example of interconverting enantiomers among natural products. Paeciloketal A showed modest antibacterial activity (MIC = 40 μg mL−1) against the marine pathogen Vibrio ichthyoenteri.
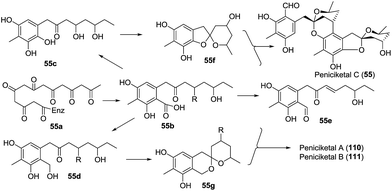
Peniciketal C (55),44 a new [5,6]-spiroketal, along with two [6,6]-spiroketal analogues, peniciketals A (110) and B (111) were isolated. Peniciketal C (55) features a fully substituted phenyl ring bearing a rare [5,6]-spiroketal core and an oxa-bicyclo[3.3.1]nonane skeleton, and peniciketal C exhibited selective effects on HL-60 cells with an IC50 value of 4.5 μM. A plausible biogenetic pathway for peniciketal C was proposed (Scheme 10). The common precursor 55a, derived from the biotransformation of the acetyl malonyl and malonyl CoA, was methylated and cyclized to afford intermediate 55b. Compound 55b was further transformed into 55c, 55d, and 55e through different reaction pathways. Compound 55c produced the spiroketal 55f, which reacted with 55e to afford peniciketal C through electrophilic aromatic substitution and ketal formation. A similar approach was proposed for the biosynthesis of peniciketals A and B.
2.3 [6,6]-Spiroketals
The newly isolated [6,6]-spiroketals are listed in Table 3, and their chemical structures are shown in Fig. 6 and 7.| Entries | Compound names/number | Isolation source | Structural elucidation | Structural classification | Bioactivity | Ref. |
|---|---|---|---|---|---|---|
| 1 | Alotaketals C, D and E (76–78) | Specimens of the sponge Phorbas sp. | HRMS (ESI), UV, 1D- and 2D-NMR | Sesterterpenoid | An activator of cAMP signaling in HEK293 cells; potent inhibitors of HIV | 52 and 53 |
| 2 | 3-Methoxyaplysiatoxin (79) and 3-methoxydebromo-aplysiatoxin (80) | The marine cyanobacterium Trichodesmium erythraeum | HRMS (ESI), UV, 1D- and 2D-NMR | Macrolides | Significant anti-CHIKV activities | 54 |
| 3 | Avermectin analogue (81–82) | A mutant Streptomyces avermectinius strain | HRMS (ESI), IR, UV, 1D- and 2D-NMR | Macrolides | Moderate cytotoxicities | 55 |
| 4 | Didemnaketals D–G (83–86) | A Red Sea Ascidian Didemnum species | HRMS (ESI), IR, UV, 1D- and 2D-NMR | Terpenoids | Moderate activity against CDK5, CK1, DyrK1A, and GSK3 kinases; antimicrobial activity; potent antimicrobial activity | 56 and 57 |
| 5 | Ganoapplanin (6) | Ganoderma applanatum | HRMS (ESI), IR, 1D- and 2D-NMR, ECD, XRD | Polycyclic meroterpenoid | Moderate inhibitory activities against TTCC | 12 |
| 6 | Gombaspiroketals A–C (87–89) | The Korean marine sponge Clathria gombawuiensis | HR MS (FAB), IR and UV, 1D- and 2D-NMR, CD, ECD | Tetracyclic sesterterpenes | Moderate cytotoxicity; antibacterial activity; weak inhibition of Na+/K+–ATPase and isocitrate lyase | 59 |
| 7 | Griseusins E–G and derivatives (90–95) | A culture of Streptomyces sp. IFM 11307; a Yunnan alkalophilic actinomycete Nocardiopsis sp. YIM DT266 | HRMS (ESI), UV, IR, 1D- and 2D-NMR; CD, XRD | Pyranonaphthoquinones | Strong cytotoxicity; antibacterial activity in vitro | 60 and 61 |
| 8 | Leonuketal (5) | The aerial parts of the plant Leonurus japonicas | HRMS (ESI), IR, 1D- and 2D-NMR, ECD, TDDFT method | Tetracyclic diterpenoid | Significant vasorelaxant activity against KCl-induced contraction of rat aorta | 11 |
| 9 | Limaol A (96) | The benthic marine dinoflagellate Prorocentrum lima | HRMS (ESI), IR, 1D- and 2D-NMR, Mosher's method | Polyketide | Moderate cytotoxicity | 62 |
| 10 | Neomaclafungins A–I (97–105) | The fermentation broth of Actinoalloteichus sp. NPS702 | HRMS (ESI-TOF), UV, IR, 1D- and 2D-NMR | Macrolide | Strong antifungal activity | 63 |
| 11 | 21-Hydroxy-oligomycin C (7) and 40-hydroxy-oligomycin B (106) | Streptomyces sp. AS5351 and Streptomyces sp. AS5339v11 | HRMS (ESI), 1D- and 2D-NMR | Macrolide | Inhibitors of K-Ras; moderate cytotoxicity | 13 |
| 12 | Peneciraistins A–C (107–109) | The saline soil derived fungus Penicillium raistrickii; Penicillium lividun; Penicillium thomii | HRMS (ESI), IR, UV, 1D- and 2D-NMR, CD | Benzannulate spiroketal | Radical scavenging activities against DPPH; significant cytotoxicity; anticancer activity | 64–66 |
| 13 | Peniciketals A–B (110–111) | The saline soil derived fungus Penicillium raistrichii | HRMS (ESI), IR, UV, 1D- and 2D-NMR, XRD, ECD | Moderate cytotoxicity | 44 | |
| 14 | Penicophenone A (112) | The culture broth of Penicillium commune | HRMS (ESI), IR, UV, 1D- and 2D-NMR, ECD | Phenone derivatives | — | 67 |
| 15 | Peniphenone A (113) | A mangrove fungus Penicillium dipodomyicola HN4-3A | HRMS (EI), UV, 1D- and 2D-NMR, XRD, CD, ECD | Benzannulated spiroketal | — | 68 |
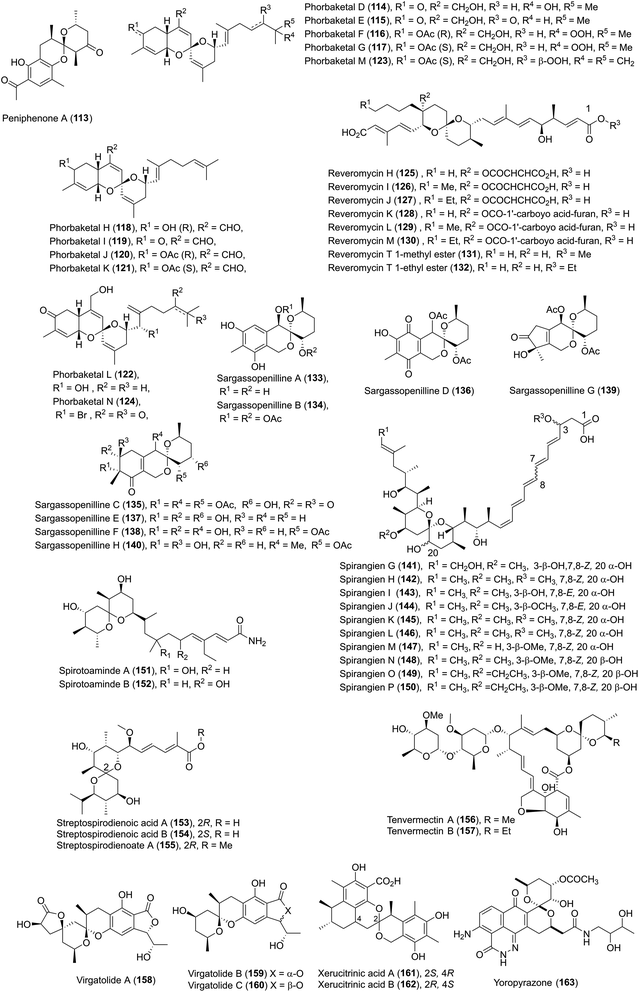
| 16 | Phorbaketals D–N (114–124) | A Korean marine sponge Monanchora sp.; a marine sponge of the genus Phorbas | HRMS (FAB), IR, UV, 1D- and 2D-NMR, CD, Mosher's method | Sesterterpenoid | Cytotoxicity | 69 and 70 |
| 17 | Reveromycins H–M (125–130) | Australian Streptomyces spp. | HRMS (ESI), 1D- and 2D-NMR | Polyketide | Moderate antifungal activities | 71 |
| 18 | Reveromycin T 1-methyl ester (131) and reveromycin T 1-ethyl ester (132) | Add alcohol to the culture broth of Streptomyces reveromyceticus SN-593 | HRMS (ESI), UV, 1D- and 2D-NMR, Mosher's method | Polyketide | Cytotoxic activities; moderate activity against tsFT210 cells | 72 |
| 19 | Sargassopenillines A–H (133–140) | The alga-derived fungi Penicillium thomii KMM 4645 and Penicillium lividum KMM 4663 | MS (ESI), HRMS (ESI), IR, UV, 1D- and 2D-NMR, Mosher's method, CD | — | Cytotoxicity; radical scavenging activity; the transcriptional activity of the oncogenic nuclear factor AP-1 | 73 and 74 |
| 20 | Spirangiens G–P (141–150) | The myxobacterium Sorangium cellulosum | HRMS (ESI), UV, 1D- and 2D-NMR, XRD, Murata's method | Polyketide | High cytotoxicities | 75 |
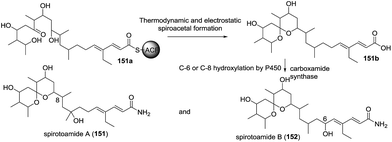
| 21 | Spirotoamides A and B (151–152) | A microbial metabolite fraction library of Streptomyces griseochromogenes JC82-1223 | HRMS (ESI), IR, XRD | Polyketide | — | 76 |
| 22 | Streptospirodienoic acids A (153)/B (154), streptospirodienoate A (155) | The fermentation broth of Streptomycetes sp. (no. 0950134) | MS (ESI-TOF), HRMS (ESI), 1D- and 2D-NMR, XRD | Polyketide | Moderate cytotoxic activity | 77 |
| 23 | Tenvermectins A and B (156–157) | MHJ1011 strain from Streptomycin avermitilis G8-17 | MS, HRMS (ESI), 1D- and 2D-NMR | Macrolide | Insecticidal activity | 78 |
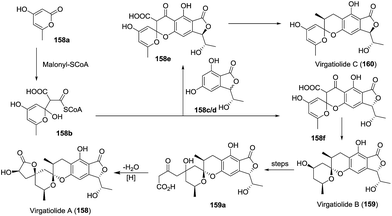
| 24 | Virgatolides A, B, and C (158–160) | The plant endophytic fungus Pestalotiopsis virgatula | HRMS (ESI), 1D- and 2D-NMR, XRD | Benzannulated spiroketals | Modest cytotoxicity | 79 |
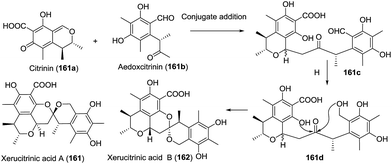
| 25 | Xerucitrinic acids A and B (161–162) | The mushroom Xerula sp. BCC56836 | HRMS (ESI), IR, UV, 1D- and 2D-NMR | Polyketide | Weak cytotoxicity; antibacterial activity | 80 |
| 26 | Yoropyrazone (163) | Streptomyces sp. IFM 11307 | HRMS (ESI), IR, UV, 1D- and 2D-NMR, CD | Naphthopyridazone | Moderate TRAIL resistance-overcoming activity against AGS cells | 81 |
In the investigation of biologically active metabolites from Red Sea marine organisms, Youssef and coworkers isolated four new spiroketals, i.e., didemnaketals D–G (83–86) in 2014.56,57 These new compounds are different from the previously reported didemnaketals A–C by Faulkner and coworkers,58 although the molecular skeleton was not obviously changed. In contrast to didemnaketal A and B,58 which exhibit anti-HIV activity, these compounds show no activity against HIV. However, didemnaketals D and E showed moderate activity against CDK5, CK1, DyrK1A, and GSK3 kinases and moderate antimicrobial activity against Staphylococcus aureus and Bacillus subtilis. Didemnaketal F displayed potent antimicrobial activity against E. coli and C. albicans and moderate activity against HeLa cells (IC50 = 49.9 μM), while didemnaketal G exhibited moderate activity against HeLa cells (IC50 = 14.0 μM).
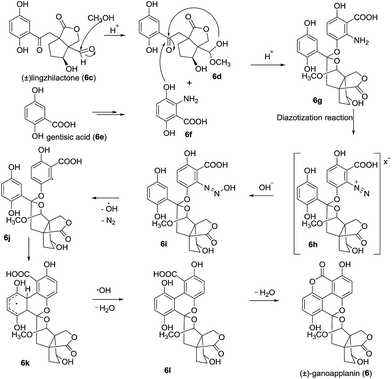
In 2016, ganoapplanin (6) was isolated as a mixture of two enantiomers in unequal amounts and possesses an unprecedented dioxaspirocyclic skeleton containing a 6/6/6/6 tetracyclic system and an unusually flabellate tricycle motif.12 Biological activity studies showed that (±)-ganoapplanin (6), (+)-ganoapplanin (6a), and (−)-ganoapplanin (6b) exhibited moderate inhibitory activities against T-type voltage-gated calcium channels (TTCC) with IC values of 36.6 μM, 51.5 μM, and 56.4 μM, respectively. These findings suggested the strong possibility of a TTCC inhibitor from Ganoderma species. Its biosynthetic approach was shown in Scheme 12. Lingzhilactone (6c) was converted to its hemiacetal 6d, which was reacted with the gentisic acid derivative 6f to afford the spiroketal 6g. Subsequent diazotization reaction and Gomberg–Bachmann reaction gave the precursor 6l, and an intermolecular esterification of 6l furnished ganoapplanin.
In the ongoing search for bioactive compounds from Korean water sponges, Shin and coworkers isolated three tetracyclic sesterterpenes spiroketals, i.e., gombaspiroketals A–C (87–89).59 They exhibited moderate cytotoxicity against the A549 and K562 cell lines, antibacterial activity against ATCC 6538p, ATCC 6633, ATCC 14028, ATCC 35270, NBRC 3851, and NBRC 12708, and weak inhibition of Na+/K+–ATPase and isocitrate lyase.
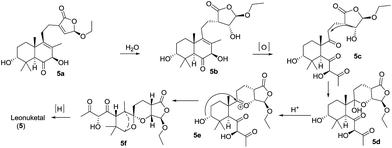
Leonuketal (5), a complex spiroketal diterpenoid, was isolated by Peng et al. in 2015.11 It features an unprecedented tetracyclic skeleton incorporating a bridged spiroketal moiety fused with a ketal-γ-lactone unit. It displayed significant vasorelaxant activity (EC50 = 2.32 μM) against KCl-induced contraction of the rat aorta. A possible biosynthesis pathway of leonuketal was proposed (Scheme 13). Water would react with an intermediate (5a) to give compound 5b, in which the double bond in the six-membered cycle could undergo selective cleavage to give triketone 5c. The spiroketalization and then selective reduction ketone of 5f could afford leonuketal.
Phorbaketals D–K (114–121) and phorbaketals L–N (122–124) were isolated by Kang and coworkers in 2013 and in 2014, respectively.69,70 Phorbaketals E (115), F (116), H (118), and I (119) exhibited weak cytotoxicity against the A498 human renal cancer cell line, while phorbaketal N (124) showed potent cytotoxicity against human pancreas cancer cells.
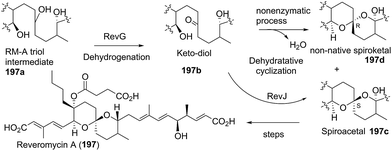
Six rare reveromycin class spiroketals were isolated by Capon et al. using HPLC-DAD-MS and HPLC-DAD-SPE-NMR methodology, and named reveromycins H–M (125–130).71 Reveromycins K–M showed moderate antifungal activities.
In the search for the biosynthetic gene cluster of reveromycin A using reveromycin T as an intermediate, two new derivatives of reveromycin T, reveromycin T 1-methyl ester (131) and 1-ethyl ester (132), were obtained through adding alcohol to the culture broth of Streptomyces reveromyceticus SN-593.72 This alcohol-added fermentation may be a potent approach to obtain new metabolites of active products containing an acid group.
Sargassopenillines A–G (133–139), were isolated in 2014.73 Two years later, sargassopenilline H (140) was also ecludicated.74 Bioactivity investigation showed that sargassopenilline A (133) possessed radical scavenging activity against DPPH (IC50 = 100 μM), sargassopenilline C (135) inhibited the transcriptional activity of the oncogenic nuclear factor AP-1 (IC50 = 15 μM), and sargassopenilline E (137) exhibited cytotoxicity against splenocytes (IC50 = 38 μM) and radical scavenging activity against DPPH (IC50 = 30 μM). Sargassopenilline H (140) in non-cytotoxic concentrations inhibited the colony formation of the RPMI-7951 and MDA-MB-231 cell lines.
Three new [6,6]-spiroketal polyketides, named streptospirodienoic acids A (153) and B (154) and streptospirodienoate A (155), were isolated.77 Notably, the authors excluded the possibility that streptospirodienoate A, a methyl esterified derivative of streptospirodienoic acid A (153) at the terminal acid, was an artefact. However, streptospirodienoate A (155) showed moderate cytotoxic activity against HCT-116 cells (IC50 = 5.2 μM), while streptospirodienoic acids A and B showed no activity.
Ten new spirangiens, designated spirangiens G–P (141–150), were isolated by Kalesse and coworkers in 2015.75 Most of them showed significant cytotoxicity against the KB-3-1 cell line and against untransformed cells (L-929) in the relatively low nanomolar range.
In the process of isolating structurally novel metabolites and investigating their bioactivities based on the spectral database of the fraction library method, Osada and coworkers identified two new [6,6]-spiroacetal polyketides, spirotoamides A (151) and B (152).76 Based on the biosynthetic approaches to tautomycin and avermectin and some experimental results, a possible pathway was proposed (Scheme 14). Dihydroxyl ketone precursor (151a) was first converted into a thermodynamically and electrostatically preferred spiroketal (151b), which was transformed to spirotoamides A and B through amidation with carboxamide synthase and selective hydroxylation by P450.
In the search for bioactive natural products from Thai microorganisms, Sadorn et al. isolated xerucitrinic acids A (161) and B (162).80 The preliminary bioassay indicated that xerucitrinic acid A exhibited moderate anti-Bacillus cereus activity (MIC 12.5 μg mL−1). Based on the citrinin moiety produced in various fungal genera, a plausible biosynthetic approach was proposed (Scheme 15). 1,6-Conjugate addition between citrinin (161a) and another precursor aedoxcitrinin (161b) gave the adduct 161c, which was selectively reduced and cyclized to afford xerucitrinic acids A and B.
In 2011, Ishibashi reported four new pyranonaphthoquinones, named (+)-griseusin E (90), (+)-methyl ester of 4′-deacetyl-griseusin B (91), (+)-4′-deacetyl-griseusin A (92) and (+)-4′-deacetyl-griseusin B (93).60 Four natural products showed significant ability to overcome tumour necrosis factor-related apoptosis-inducing ligand resistance in human gastric adenocarcinoma cell lines. One year later, two analogues, named griseusin F (94) and G (95), were isolated by Wen and Zhu.61 Both compounds feature a previously undescribed C23 polyketide skeleton. Griseusins F and G exhibited strong cytotoxicity against the B16, MDA-MB435S, CFPAC-1, ACHN, and HCT-116 human cancer cell lines (IC50 0.37–0.82 μM) and antibacterial activity against Staphylococcus aureus ATCC 29213 with MIC 0.91 and 0.80 μg mL−1, respectively.
As a new member of the griseusin family, yoropyrazone (163), was isolated by Ishibashi.81 In contrast to other griseusins, yorophenazone bears a usual naphthoiminoquinone, a naphthoquinone and an amide chain containing a vicinal diol, for which stereochemistries have not been determined. It is the first naturally occurring microbial naphthopyridazone. An investigation of the TRAIL resistance-overcoming activity of yoropyrazone in human gastric adenocarcinoma (AGS) cell lines showed moderate cytotoxic activity.
Very recently, Sun reported the isolation and structural elucidation of two new avemectin analogues, 81 and 82;55 bioassay revealed that they exhibited moderate cytotoxicities in vitro against MG-63, B16, and Saos-2 cell lines.
Ras protein is important in cell growth, proliferation and differentiation, and inhibition of K-Ras plasma membrane localization would be an effective approach in the treatment of cancer. Therefore, the discovery and development of new chemical scaffolds to mislocalize oncogenic mutant K-Ras remain a challenge. Very recently, Capon and colleagues isolated two new 26-membered macrocyclic lactone oligomycin derivatives, 21-hydroxy-oligomycin C (7) and 40-hydroxy-oligomycin B (106).13 Preliminary bioactivity screening results confirmed that 21-hydroxy-oligomycin C and 40-hydroxy-oligomycin B are potent inhibitors of K-Ras and exhibit moderate cytotoxicity to colon cancer cell line SW620. These results pave the way for the development of new probes to treat K-Ras-related cancers.
Screening of the fermentation extract of strain NPS702 revealed nine new analogues of maclafungin, neomaclafungins A–I (97–105), which possess a 26-membered macrolide.63 The presence of alkane or alkanol branches on the [6,6]-spiroketal skeleton are the major differences in their chemical structures. Neomaclafungins A–I showed strong antifungal activity against Trichophyton mentagrophytes (ATCC 9533) with MIC values between 1 and 3 μg mL−1in vitro.
Gene replacement of polyketide synthase (PKS) could serve as an alternative approach to obtaining some biologically important natural products. Using this method, Zheng and coworkers discovered two new avemectin analogues, named tenvermectins A (156) and B (157).78 They showed better bioactivities against Tetranychus cinnabarinus and Bursaphelenchus xylophilus than avermectin, ivermectin, and milbemycin, respectively.
In an ongoing effort to find structurally diverse natural products with interesting bioactivity, Zhu and coworkers isolated peniciketals A–B (110–111), two new [6,6]-spiroketals bearing a benzo-fused 2,8-dioxabicyclo[3.3.1]nonane moiety.44 Peniciketals A and B showed moderate cytotoxicity against HL-60 cells (IC50 = 3.2 and 6.7 μM, respectively). Their possible biosynthesis route is shown in Scheme 10.
In 2016, Zhang and coworkers reported the isolation of penicophenone A (112).67 A preliminary 11β-HSD1 assay of this compound was carried out, but unfortunately, no activity was observed. Its biosynthesis was proposed to proceed through a similar pathway to that of peniphenone A (see below).
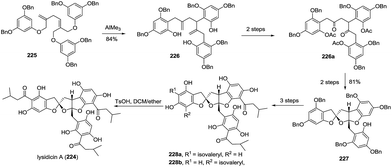
Peniphenone A (113), bearing a rare benzannulated [6,6]-spiroketal, was isolated.68 Based on the co-isolated compound 2,4-dihydroxy-3,5-dimethylacetophenone (113a), a possible biosynthetic pathway was proposed (Scheme 16). 3,6-Dimethyl-4-hydroxy-2-pyrone derivative (113b), derived from a biotransformation of acetyl CoA, reacted with ortho-quinone methide 113d from 2,4-dihydroxy-3,5-dimethylacetophenone (113c) to afford the intermediate 113e, which further transformed to produce peniphenone A. A similar transformation could explain the possible biosynthesis of its analogues, such as penicophenone A and sargassopenillines A–G.
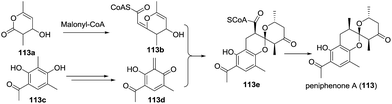 | ||
| Scheme 16 The possible biosynthesis route of (±)-peniphenone A, penicophenone A, and sargassopenillines. | ||
In 2011, Che and coworkers isolated three benzannulated spiroketals, virgatolides A–C (158–160).79 These novel compounds feature a 3,4,5,6-tetrahydrospiro[chroman-2,2′-pyran] core. Remarkably, virgatolide A contains two γ-lactone units, one spiralled with the spiroketal skeleton and the other fused with the substituted benzyl ring. Notably, virgatolide A is the first example of the unique benzannulated [6,6]-spiroketal bearing γ-lactone skeleton. The preliminary bioactivity investigation indicated that virgatolides A, B, and C exhibited modest cytotoxicity against HeLa cells with IC50 values of 19.0, 22.5 and 20.6 μM, respectively. Based on the co-isolated compounds pestaphthalide A and B, the biosynthetic pathway to these spiroketals was also proposed (Scheme 17). 3,6-Dimethyl-4-hydroxy-2-pyrone (158a) was reacted with demethyl pestaphthalide A (158c) and B (158d) to produce the spiroketals 158e and 158f, respectively. Subsequent cascade transformations of two intermediates yielded virgatolides B and C, while virgatolide A was derived from virgatolide B through oxidation/nucleophilic reaction/lactonization/reduction processes.
2.4 [m,n]-Spiroketals
The newly isolated [m,n]-spiroketals are listed in Table 4, and their chemical structures are shown in Fig. 8.| Entries | Compound names/number | Isolation source | Structural elucidation | Structural classification | Bioactivity | Ref. |
|---|---|---|---|---|---|---|
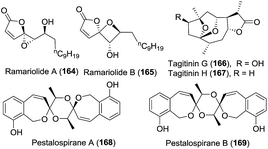
| 1 | Ramariolide A (164) | The coral mushroom Ramaria cystidiophora | ESI-QIT-MS, HRMS (ESI), 1D- and 2D-NMR, XRD, Mosher's method | Butenolide | Antimicrobial activity in vitro | 82 |
| 2 | Ramariolide B (165) | The coral mushroom Ramaria cystidiophora | HRMS (ESI), 1D- and 2D-NMR | Butenolide | — | 82 |
| 3 | Tagitinins G and H (166–167) | The aerial parts of Tithonia diversifolia | IR, 1D- and 2D-NMR, XRD | Sesquiterpene | Anti-hyperglycemic activity | 83 |
| 4 | Pestalospiranes A and B (168–169) | The endophytic fungus Pestalotiopsis virgatula | MS, IR, UV, 1D- and 2D-NMR, ECD | Benzo[c]oxepin | — | 84 |
Ramariolide A (164), containing an unusual spiro-oxiranebutenolide moiety, was isolated.82 It displayed antimicrobial activity against Mycobacterium smegmatis and Mycobacterium tuberculosis in vitro. Along with ramariolide A, ramariolide B (165) was obtained by the same group.82 Structurally, it is the second natural product isolated that bears a spirooxetanebutenolide.
Tagitinins G (166) and H (167) were isolated by Sun and coworkers in 2012.83 Tagitinin G exhibited remarkable anti-hyperglycaemic activity by glucose uptake in 3T3-L1 adipocytes without significant toxic effects at 10 μg mL−1 concentration.
In the process of the structure elucidation of natural products using HPLC-SPE-NMR, which is superior to the classical preparative-scale fractionation of extracts originating from natural sources in some cases, Jaroszewski et al. isolated two metabolites, i.e., pestalospiranes A (168) and B (169).84 These compounds feature a rare benzo-[c]-oxepin skeleton, and their highly symmetric chemical structures were assigned by a combination of NMR spectroscopy and ECD spectroscopy.
2.5 Biosynthesis of spiroketals
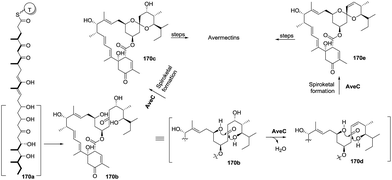
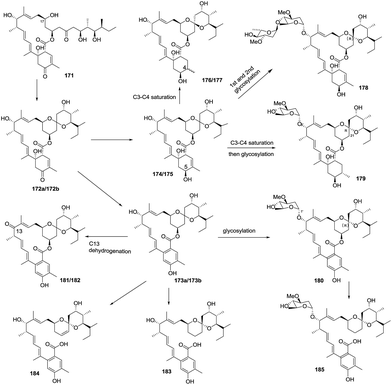
In 2014, Liu and co-workers reported nine avermectin analogues (174–182), which were all derived from a common biosynthetic intermediate 171, from the triple-mutant S. avermectinius strain VL1004 (Scheme 19).22 Interestingly, the absolute configuration of spiroatom (C-21) in some analogues was S, which is different from the previously isolated natural avermectins (i.e. R configuration at C-21). The C-21 carbons of these analogues are in the S configuration, indicating they were produced via different biosynthetic pathways and have different bioactivities than those of the C-21 R-type natural products. Moreover, these bioactivity differences indicate that the furan core and the sugar moiety in the avermactins have an important role in the activity. One year later, the same group reported three new derivatives (183–185) of 1,19-seco-avermactin from the ΔaveCDE mutant S. avermetinius strain, which were deduced from the compounds 173 and 180, respectively (Scheme 19).86
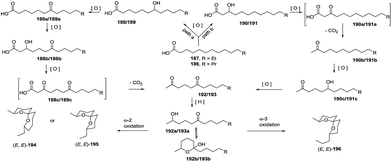
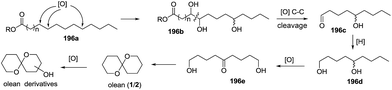
Three years later, based on the extensive experimental results, De Voss and colleagues proposed the possible biosynthetic pathway of olean and its derivatives in Bactrocera cacuminata.87 Starting from a fatty acid equivalent 196a, an enzyme-mediated C–H bond oxidation process at specific positions yielded a trioxygenated intermediate 196b with a vicinal diol moiety, which could be converted to key synthetic precursor 196c through oxidative C–C bond cleavage. This compound was transformed to olean through subsequent reduction, selective oxidation and cyclization, and the oxidation of the resulted olean afforded its derivatives (Scheme 21). Therefore, considering previously reported results, the complete biosynthetic pathway of olean and its spiroacetal derivatives was well-established. Notably, this oxidative transformation involving P450 was originally reported in insects.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio.
2 ratio.
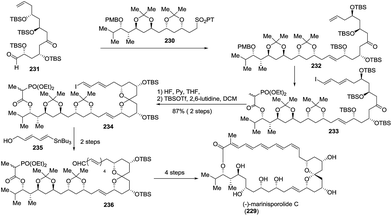
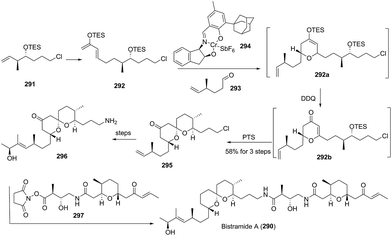
3. Total syntheses of natural spiroketals
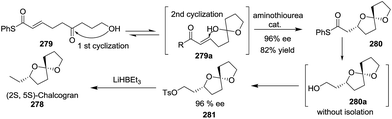
The total synthesis of natural spiroketals has been a promising field for synthetic chemists because these heterocyclic compounds possess unique structures and interesting biological activities, albeit with only small amounts, even trace amounts, isolated from nature. Consequently, access to numerous natural spiroketals has mainly relied on total synthesis via different strategies. In this section, some representative examples have been provided and correspondingly categorized into six subclasses. This review aims to emphasize the key steps for the total syntheses of spiroketals, especially synthetic methods for the construction of the spiroketal scaffold, and therefore, other chemical transformations will be discussed only briefly. Because some elegant syntheses of natural subclasses of spiroketal products have been recently reviewed by Brimble,90 Tong,91,92 Pettus,93 and Paterson,94 these previously reviewed synthetic approaches will not be introduced. Additionally, both the fragmentary synthetic studies on natural spiroketals and the preparation of natural spiroketal analogues will be neglected in this review.3.1 Synthesis based on the classical cyclization of a dihydroxyketone or its equivalent to the key spiroketal moiety
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
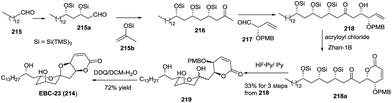
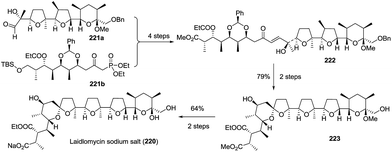
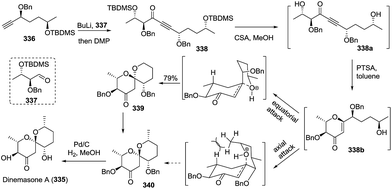
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of spirocyclic products, which were isomerized to furnish spiroketal 223 with the expected stereochemistry in 79% yield over two steps. The removal of the protected groups completed the total synthesis of laidlomycin sodium salt (Scheme 27).
1 mixture of spirocyclic products, which were isomerized to furnish spiroketal 223 with the expected stereochemistry in 79% yield over two steps. The removal of the protected groups completed the total synthesis of laidlomycin sodium salt (Scheme 27).
3.2 Based on transition metal-promoted transformation to obtain key spiroketal moiety
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
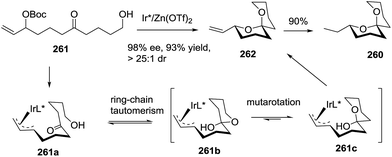
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr through ring-chain tautomerism/mutarotation approach. Hydrogenation of the resulting product gave the target molecule 260 in 90% yield (Scheme 34).
1 dr through ring-chain tautomerism/mutarotation approach. Hydrogenation of the resulting product gave the target molecule 260 in 90% yield (Scheme 34).
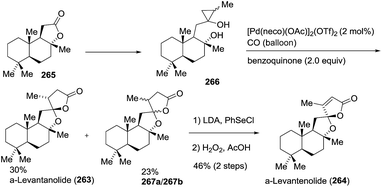
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond into the lactone ring was achieved by the treatment of a mixture of α-levantanolide and other isomers with phenylselenyl chloride, followed by oxidation with H2O2 to provide α-levantenolide (264) as a single product in 46% yield in two steps. The structure of α-levantenolide was further confirmed by X-ray diffraction (Scheme 35).
C double bond into the lactone ring was achieved by the treatment of a mixture of α-levantanolide and other isomers with phenylselenyl chloride, followed by oxidation with H2O2 to provide α-levantenolide (264) as a single product in 46% yield in two steps. The structure of α-levantenolide was further confirmed by X-ray diffraction (Scheme 35).
3.3 Based on organocatalysed transformation to obtain key spiroketal moiety
The organocatalysed asymmetric synthesis of spiroketals, especially for the corresponding synthetic precursors without active groups to interact with the reaction catalyst, has been a great challenge and remains an unclear topic in organic synthesis.112 Delightfully, this tricky issue was solved by List and co-worker in 2012. They applied the exceptionally bulky chiral imidodiphosphoric acid as an organic catalyst to induce allylic ether to form planar oxocarbenium ions and then interact with an intermediate through a rigid counter ion pair approach, resulting in the highly stereoselective construction of spiroketals.83.4 Based on the cycloaddition reaction to obtain the key spiroketal moiety
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
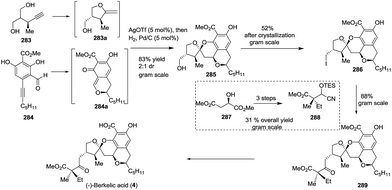
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr value, containing four rings with five stereocentres, in only one step, and produced 2.4 g of the isolated product. To introduce the lateral chain and avoid a tedious oxidation process, the new umpolung synthesis strategy was applied. The treatment of the primary iodide 286, which was obtained from alcohol 285 in gram scale, with cyanohydrin 288 afforded the precursor 289, of which the benzylic ester was removed to complete the total synthesis of (−)-berkelic acid in a seven-step linear sequence (Scheme 40).
1 dr value, containing four rings with five stereocentres, in only one step, and produced 2.4 g of the isolated product. To introduce the lateral chain and avoid a tedious oxidation process, the new umpolung synthesis strategy was applied. The treatment of the primary iodide 286, which was obtained from alcohol 285 in gram scale, with cyanohydrin 288 afforded the precursor 289, of which the benzylic ester was removed to complete the total synthesis of (−)-berkelic acid in a seven-step linear sequence (Scheme 40).
3.5 Based on the rearrangement reaction to obtain the key spiroketal moiety
3.6 Based on other novel processes to obtain the key spiroketal moiety
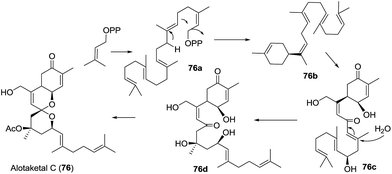
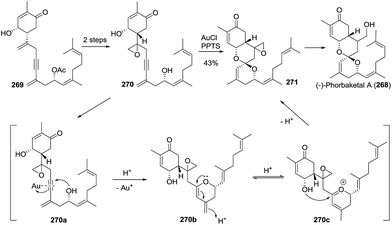
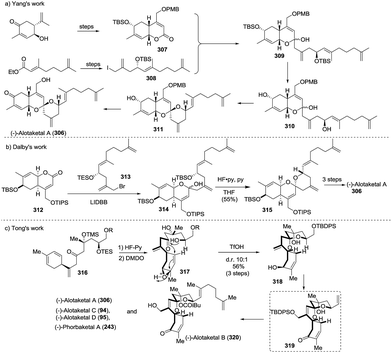
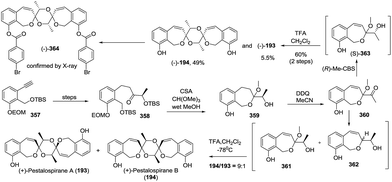
The first total synthesis of (−)-alotaketal A (306) was reported by Yang and coworkers in 2012.118 The coupling of lactone 307 (prepared from (R)-carvone) and allylic iodide 308 (prepared from ethyl acetoacetate) was carried out under Barbier conditions to afford the desired hemiacetal 309, which was desilylated using TBAF and then cyclized using PPTS to give the expected spiroketal 311 as a sole product with 40% yield over three steps. The author speculated that the electron-withdrawing inductive effect of PMB at the C-22 position is a key factor. The oxidation of allylic alcohol 311 followed by the removal of the PMB group from the compound furnished alotaketal A (Scheme 43a).
In the same year, Dalby and coworkers described a convergent approach towards (−)-alotaketal A.119 The key LiDBB-mediated coupling of the chiral lactone 312 with allyl bromide 313 afforded the hemiacetal 314, which was subjected to HF·pyridine buffered solution to give the spiroketone 315 with 55% yield, and the spirocentre was efficiently constructed through the anomerically stabilized configuration. The subsequent selective removal of the TIPS group/oxidation/selective reduction completed the total synthesis of (−)-alotaketal A (Scheme 43b).
Very recently, Tong and coworkers described a novel synthetic strategy for the collective synthesis of (−)-alotaketals A–D and (−)-phorbaketal A.120 The sequential treatment of 316 with HF-pyridine and DMDO afforded the epoxide 317, which was subjected to TfOH to promote the designed cascade reaction of vinyl epoxy δ-ketoalcohols, resulting in the desired tricyclic spiroketal 318 in 56% yield on a 4.5 g scale, along with the minor isomer. Interestingly, the isomerization product of the exo-double bond was not observed. The adjustment of the oxidation state and the installation of some functional groups afforded the common intermediate 319, which was transformed to (−)-alotaketals A (306), B (320), C (94), D (95) and (−)-phorbaketal A (243) based on different transformations (Scheme 43c).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
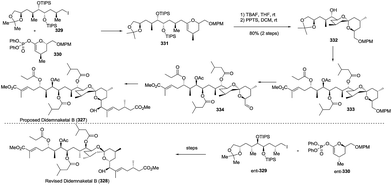
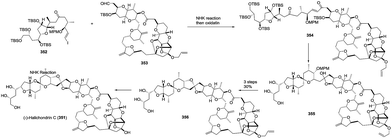
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The enlargement of a side chain, installation of ester, deprotection and oxidation gave the C1–C21 aldehyde segment 333, which was coupled with the C22–C28 side chain under Nozaki–Hiyama–Kishi (NHK) reaction conditions to complete the total synthesis of the proposed didemnaketal B, 327. Unfortunately, the spectral data of the synthetic didemnaketal B did not match the spectral data of the reported didemnaketal B, 328. After careful examination of the original literature and comparison of the spectral data of the key intermediate to elucidate the chemical structure of didemnaketal B, the author proposed that the absolute configuration of the C10–C20 spiroketal core might be erroneously assigned. Therefore, starting from ent-329 and ent-330, the natural didemnaketal B was synthesized by following a similar approach toward the proposed didemnaketal B, resulting in the real structure of the didemnaketal B (Scheme 45).
1). The enlargement of a side chain, installation of ester, deprotection and oxidation gave the C1–C21 aldehyde segment 333, which was coupled with the C22–C28 side chain under Nozaki–Hiyama–Kishi (NHK) reaction conditions to complete the total synthesis of the proposed didemnaketal B, 327. Unfortunately, the spectral data of the synthetic didemnaketal B did not match the spectral data of the reported didemnaketal B, 328. After careful examination of the original literature and comparison of the spectral data of the key intermediate to elucidate the chemical structure of didemnaketal B, the author proposed that the absolute configuration of the C10–C20 spiroketal core might be erroneously assigned. Therefore, starting from ent-329 and ent-330, the natural didemnaketal B was synthesized by following a similar approach toward the proposed didemnaketal B, resulting in the real structure of the didemnaketal B (Scheme 45).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
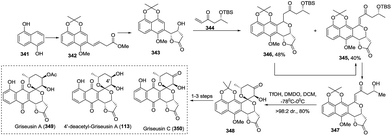
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr through TfOH-controlled cascade si-facial selective epoxidation/cyclization. The precursor 348 was concisely transformed into the natural griseusin A (349), 4′-deacetyl-griseusin A (113), and griseusin C (350) in a 1–3 step chemical transformation (Scheme 47).
1 dr through TfOH-controlled cascade si-facial selective epoxidation/cyclization. The precursor 348 was concisely transformed into the natural griseusin A (349), 4′-deacetyl-griseusin A (113), and griseusin C (350) in a 1–3 step chemical transformation (Scheme 47).
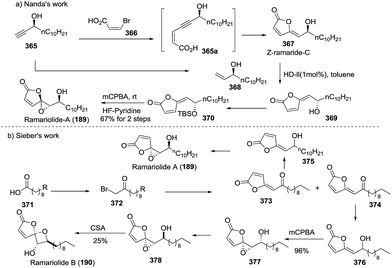
At the same time, the first total synthesis of ramariolides A (189) and B (190) was reported by Sieber and coworkers.129 The commercially available fatty acid 371 was first transformed into the corresponding α-bromoketone 372 through three consecutive conversions, and the subsequent Wittig reaction with maleic anhydride afforded the (E)-butenolide moiety 373 as a major isomer, which was reduced by NaBH4 to yield the allylic alcohol 375. The subsequent Sharpless epoxidation accomplished the total synthesis of ramariolide A with 35% yield and a modest 55% ee. In parallel, the oxidation of the allylic alcohol 376 derived from a minor (Z)-butenolide moiety 374 with mCPBA yielded the syn-configuration α-hydroxy epoxide 377, and the subsequent inversion of configuration of the hydroxyl group and then CSA-promoted cyclization completed the first total synthesis of ramariolide B with low 25% yield (Scheme 50b).
4. Summary and perspectives
From the simple insect pheromone olean to the sophisticated anticancer agent spongistatin, from pesticide avermectins to T-type voltage-gated calcium channel inhibitor ganoapplanin, natural spiroketals isolated from various sources including plants, animals, and microbes have shown broad and interesting bioactivities, providing great possibilities for drug discovery and design, structure optimization, and functional reassignment, which are important topics in scientific research. As such, spiroketals have attracted substantial attention from the relevant chemists, and amazing achievements have consequently been made due to the relentless endeavours of researchers in recent years. These achievements include the following: (1) new chemical structures have been discovered based on bioactivity-oriented isolation or the combination of multiple methods and techniques, such as LS-MS-NMR, HPLC-DAD-MS and HPLC-DAD-SPE-NMR; (2) the novel bioactivities of natural spiroketals have been explored; (3) numerous convenient and efficient synthetic routes toward natural spiroketals have been reported from synthetic communities; (4) the biosynthesis of spiroketals has been a blooming topic, especially polyketide-related spiroketals, which have been extensively investigated by many biologists and chemists.Nevertheless, the investigation of natural spiroketals remains in its infancy because many spiroketal-related issues that need to be solved are still being investigated. (1) Although novel chemical structures have been discovered, the isolation of only trace amounts and the scarce resources in nature have limited widespread bioassays and other functional studies. Therefore, how to efficiently and practically obtain highly bioactive spiroketals is a central issue that needs to be solved, but it is a great challenge. Total synthesis and biosynthesis may be two preferred approaches. Total synthesis could solve the problem of scarce spiroketals by chemical transformation, and would also provide plentiful analogues of natural spiroketals. At the same time, biosynthesis provides the simplest and the most efficient approach to the synthesis of natural products in some cases, and it is of great benefit to reveal the mystery of the biotransformation of spiroketals. These syntheses will facilitate bioassays, drug design, SAR studies, and discovery of new lead compounds. Gratifyingly, some new synthetic techniques have also been applied in the total synthesis of sophisticated spiroketal natural products. For example, the total syntheses of spiranien A and spiranien A methyl ester were achieved by flow chemistry technologies.130 (2) The bioactivity model of spiroketals needs to be further clarified, because a deep understanding of the origin of bioactivity could provide a platform for the design of potential lead compounds and drug candidates for the treatment of a variety of human diseases. (3) Structurally, due to the presence of two oxygen atoms interacting with a transition metal ion such as Fe, Zn, or Cu, even Li, K, or Na, spiroketals could be used as ideal probes for protein interaction and the investigation of other biological process, providing many chances for chemical biology.131,132 (4) The easy epimerization of the spiro-atom stereocentre is another challenge for drug metabolism, drug development and the design of total synthetic strategies. (5) The biosynthesis of spiroketals is still in its infancy, since only a few types of spiroketals have been explored. For example, polyketide cyclization and P450 function in the formation of spiroketals, and numerous spiroketal biotransformations remain unknown.
The spiroketals have therefore provided wonderful opportunities for further scientific research, and we believe that the related investigations will lead to the discovery and development of novel functional spiroketal molecules. These can be widely applied in medicinal chemistry, material chemistry, chemical biology, and other fields, resulting in a number of achievements in the near future.
5. Conflicts of interest
There are no conflicts to declare.6. Acknowledgements
We gratefully acknowledge financial support from the NSFC (Grant Nos. 21772076, 21272097, 21290181, and 21372104) and PCSIRT of MOE (No. IRT-15R28).7. Notes and references
- R. G. Carter and D. L. Kuiper, Science of Synthesis, Stereoselective Synthesis, ed. J. G. De Vries, G. A. Molander and P. A. Evans, 2011, vol. 2, pp. 863–914 Search PubMed.
- J. A. Palmes and A. Aponick, Synthesis, 2012, 44, 3699–3721 CrossRef CAS.
- R. Quach, D. P. Furkert and M. A. Brimble, Org. Biomol. Chem., 2017, 15, 3098–3104 CAS.
- S. V. Ley, L. G. Milroy and R. M. Myers, Science of Synthesis, 2007, vol. 29, pp. 613–689 Search PubMed.
- M. A. Brimble and L. A. Stubbing, Synthesis of Saturated Oxygenated Heterocycles I, Topics in Heterocyclic Chemistry, ed. J. Cossy, Springer-Verlag, Berlin Heidelberg, 2014, vol. 35, pp. 189–267 Search PubMed.
- F. Perron and K. F. Albizati, Chem. Rev., 1989, 89, 1617–1661 CrossRef CAS.
- J. E. Aho, P. M. Pihko and T. K. Rissa, Chem. Rev., 2005, 105, 4406–4440 CrossRef CAS PubMed.
- I. Čorić and B. List, Nature, 2012, 483, 315–319 CrossRef PubMed.
- W. C. Campbell, Angew. Chem., Int. Ed., 2016, 55, 10184–10189 CrossRef CAS PubMed.
- A. A. Stierle, D. B. Stierle and K. Kelly, J. Org. Chem., 2006, 71, 5357–5360 CrossRef CAS PubMed.
- L. Xiong, Q.-M. Zhou, Y. Zou, M.-H. Chen, L. Guo, G.-Y. Hu, Z.-H. Liu and C. Peng, Org. Lett., 2015, 17, 6238–6241 CrossRef CAS PubMed.
- L. Li, H. Li, X.-R. Peng, B. Hou, M.-Y. Yu, J.-R. Dong, X.-N. Li, L. Zhou, J. Yang and M.-H. Qiu, Org. Lett., 2016, 18, 6078–6081 CrossRef CAS PubMed.
- A. A. Salim, L. Tan, X.-C. Huang, K.-J. Cho, E. Lacey, J. F. Hancock and R. J. Capon, Org. Biomol. Chem., 2016, 14, 711–715 CAS.
- D. J. Atkinson and M. A. Brimble, Nat. Prod. Rep., 2015, 32, 811–840 RSC.
- Q. Zheng, Z. Tian and W. Liu, Curr. Opin. Chem. Biol., 2016, 31, 95–102 CrossRef CAS PubMed.
- Y. K. Booth, W. Kitching and J. J. De Voss, Nat. Prod. Rep., 2009, 26, 490–525 RSC.
- L.-Y. Wang, J.-J. Qin, Z.-H. Chen, Y. Zhou, W. Tang, J.-P. Zuo and W.-M. Zhao, J. Nat. Prod., 2017, 80, 1102–1109 CrossRef CAS PubMed.
- M. Li, J. Xiong, Y. Huang, L.-J. Wang, Y. Tang, G.-X. Yang, X.-H. Liu, B.-G. Wei, H. Fan, Y. Zhao, W.-Z. Zhai and J.-F. Hu, Tetrahedron, 2015, 71, 5285–5295 CrossRef CAS.
- W.-W. Jiang, Y.-C. Liu, Z.-J. Zhang, Y.-C. Liu, J. He, J. Su, X. Cheng, L.-Y. Peng, L.-D. Shao, X.-D. Wu, J.-H. Yang and Q.-S. Zhao, Fitoterapia, 2016, 109, 155–161 CrossRef CAS PubMed.
- L. Wang, X.-L. Jiang, W.-M. Zhang, F. Li, A.-A. Khan, X. Liu, K. Yu and M.-K. Wang, Phytochemistry, 2017, 136, 125–132 CrossRef CAS PubMed.
- Y. K. Booth, W. Kitching and J. J. De Voss, ChemBioChem, 2011, 12, 155–172 CrossRef CAS PubMed.
- P. Sun, Q. Zhao, H. Zhang, J. Wu and W. Liu, ChemBioChem, 2014, 15, 660–664 CrossRef CAS PubMed.
- P. Phainuphong, V. Rukachaisirikul, K. Tadpetch, Y. Sukpondma, S. Saithong, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Phytochemistry, 2017, 137, 165–173 CrossRef CAS PubMed.
- S.-Y. Cheng, K.-J. Huang, S.-K. Wang and C.-Y. Duh, Mar. Drugs, 2011, 9, 1469–1476 CrossRef CAS PubMed.
- D.-W. Zhang, X. Liu, D. Xie, R. Chen, X.-Y. Tao, J.-H. Zou and J. Dai, Chem. Pharm. Bull., 2013, 61, 576–580 CrossRef CAS PubMed.
- L. Hu, H. Zhu, L. Li, J. Huang, W. Sun, J. Liu, H. Li, Z. Luo, J. Wang, Y. Xue, Y. Zhang and Y. Zhang, Sci. Rep., 2016, 6, 27588 CrossRef CAS PubMed.
- M. Kubo, Y. Nishikawa, K. Harada, M. Oda, J.-M. Huang, H. Domon, Y. Terao and Y. Fukuyama, J. Nat. Prod., 2015, 78, 1466–1469 CrossRef CAS PubMed.
- N. Nguyen, T. Ha, S. Choi, S. Eum, C. H. Lee, T. T. Buch and W. K. Oh, Phytochemistry, 2016, 130, 291–300 CrossRef CAS PubMed.
- A. Grudniewska, S. Hayashi, M. Shimizu, M. Kato, M. Suenaga, H. Imagawa, T. Ito, Y. Asakawa, S. Ban, T. Kumada, T. Hashimoto and A. Umeyama, Org. Lett., 2014, 16, 4695–4697 CrossRef CAS PubMed.
- H. Wang, J. Hong, J. Yin, H. R. Moon, Y. Liu, X. Wei, D.-C. Oh and J. H. Jung, J. Nat. Prod., 2015, 78, 2832–2836 CrossRef CAS PubMed.
- Y.-Y. Fan, H. Zhang, Y. Zhou, H.-B. Liu, W. Tang, B. Zhou, J.-P. Zuo and J.-M. Yue, J. Am. Chem. Soc., 2015, 137, 138–141 CrossRef CAS PubMed.
- Y.-L. Zhang, L.-L. Jiang, T.-S. Xiao, S.-Q. Chen and Y.-B. Li, J. Asian Nat. Prod. Res., 2015, 17, 717–723 CrossRef CAS PubMed.
- S.-J. Wang, L. Bao, J.-J. Han, Q.-X. Wang, X.-L. Yang, H.-A. Wen, L.-D. Guo, S.-J. Li, F. Zhao and H.-W. Liu, J. Nat. Prod., 2013, 76, 45–50 CrossRef CAS PubMed.
- Q.-Q. Tao, K. Ma, L. Bao, K. Wang, J.-J. Han, W.-Z. Wang, J.-X. Zhang, C.-Y. Huang and H.-W. Liu, Planta Med., 2016, 82, 639–644 CrossRef CAS PubMed.
- G. Chianese, B.-B. Gu, F. Yang, W.-H. Jiao, Y.-W. Guo, H.-W. Lin and O. Taglialatela-Scafati, RSC Adv., 2015, 5, 63372–63376 RSC.
- T. Nogawa, N. Ogita, Y. Futamura, S. Negishi, N. Watanabe and H. Osada, J. Antibiot., 2017, 70, 705–707 CrossRef CAS PubMed.
- T. Ito, H. Ito, M. Oyama, T. Tanaka, D. Darnaedi and M. Iinuma, Phytochem. Lett., 2012, 5, 325–328 CrossRef CAS.
- B. S. Hwang, H. S. Kim, W. Yih, E. J. Jeong and J.-R. Rho, Org. Lett., 2014, 16, 5362–5365 CrossRef CAS PubMed.
- K. D. G. Wang, J. Wang, S.-S. Xie, Z.-R. Li, L.-Y. Kong and J. Luo, Tetrahedron Lett., 2016, 57, 32–34 CrossRef CAS.
- L.-L. Liu, R. Wang and Y.-P. Shi, Planta Med., 2011, 77, 1806–1810 CrossRef CAS PubMed.
- Y. Luan, H. Wei, Z. Zhang, Q. Che, Y. Liu, T. Zhu, A. Mándi, T. Kurtán, Q. Gu and D. Li, J. Nat. Prod., 2014, 77, 1718–1723 CrossRef CAS PubMed.
- L. Ma, F. Ge, C.-P. Tang, C.-Q. Ke, X.-Q. Li, A. Althammer and Y. Ye, Tetrahedron, 2011, 67, 3533–3539 CrossRef CAS.
- J. Ren, F. Zhang, X. Liu, L. Li, G. Liu, X. Liu and Y. Che, Org. Lett., 2012, 14, 6226–6229 CrossRef CAS PubMed.
- W.-Z. Liu, L.-Y. Ma, D.-S. Liu, Y.-L. Huang, C.-H. Wang, S.-S. Shi, X.-H. Pan, X.-D. Song and R.-X. Zhu, Org. Lett., 2014, 16, 90–93 CrossRef CAS PubMed.
- Y. Liu, J. Ma, Q. Zhao, C. Liao, L. Ding, L. Chen, F. Zhao and F. Qiu, J. Nat. Prod., 2013, 76, 1150–1156 CrossRef CAS PubMed.
- C. N. Diep, E. G. Lyakhova, D. V. Berdyshev, A. I. Kalinovsky, V. A. Tu, N. X. Cuong, N. H. Nam, C. V. Minh and V. A. Stonik, Tetrahedron Lett., 2015, 56, 7001–7004 CrossRef CAS.
- M. G. Phan, T. T. N. Tran, T. S. Phan, K. Matsunami and H. Otsuka, Phytochem. Lett., 2014, 9, 137–140 CrossRef CAS.
- Y. Egami, T. Wakimoto and I. Abe, Bioorg. Med. Chem. Lett., 2014, 24, 5150–5153 CrossRef CAS PubMed.
- J.-J. Lv, S. Yu, Y. Xin, R.-R. Cheng, H.-T. Zhu, D. Wang, C.-R. Yang, M. Xu and Y.-J. Zhang, Phytochemistry, 2015, 117, 123–134 CrossRef CAS PubMed.
- Y. Zang, G. Genta-Jouve, P. Retailleau, A. Escargueil, S. Mann, B. Nay and S. Prado, Org. Biomol. Chem., 2016, 14, 2691–2697 CAS.
- T. Asai, Y.-M. Chung, H. Sakurai, T. Ozeki, F.-R. Chang, K. Yamashita and Y. Oshima, Org. Lett., 2012, 14, 513–515 CrossRef CAS PubMed.
- J. Daoust, M. Chen, M. Wang, D. E. Williams, M. A. G. Chavez, Y. A. Wang, C. E. Merchant, A. Fontana, T. J. Kieffer and R. J. Andersen, J. Org. Chem., 2013, 78, 8267–8273 CrossRef CAS PubMed.
- M. Wang, I. Tietjen, M. Chen, D. E. Williams, J. Daoust, M. A. Brockman and R. J. Andersen, J. Org. Chem., 2016, 81, 11324–11334 CrossRef CAS PubMed.
- D. K. Gupta, P. Kaur, S. T. Leong, L. T. Tan, M. R. Prinsep and J. J. H. Chu, Mar. Drugs, 2014, 12, 115–127 CrossRef PubMed.
- Y. Zhang, C. Zhang, K. Wang, G. Chen and P. Sun, Chem. Biodiversity, 2017, 14, e1700054 Search PubMed.
- G. A. Mohamed, S. R. M. Ibrahim, J. M. Badr and D. T. A. Youssef, Tetrahedron, 2014, 70, 35–40 CrossRef CAS.
- L. A. Shaala, D. T. A. Youssef, S. R. M. Ibrahim, G. A. Mohamed, J. M. Badr, A. L. Risinger and S. L. Mooberry, Mar. Drugs, 2014, 12, 5021–5034 CrossRef CAS PubMed.
- B. C. M. Potts, D. J. Faulkner, J. A. Chan, G. C. Simolike, P. Offen, M. E. Hemling and T. A. Francis, J. Am. Chem. Soc., 1991, 113, 6321–6322 CrossRef CAS.
- J.-K. Woo, C.-K. Kim, S.-H. Kim, H. Kim, D.-C. Oh, K.-B. Oh and J. Shin, Org. Lett., 2014, 16, 2826–2829 CrossRef CAS PubMed.
- M. S. Abdelfattah, T. Kazufumi and M. Ishibashi, J. Antibiot., 2011, 64, 729–734 CrossRef CAS PubMed.
- Z.-G. Ding, J.-Y. Zhao, M.-G. Li, R. Huang, Q.-M. Li, X.-L. Cui, H.-J. Zhu and M.-L. Wen, J. Nat. Prod., 2012, 75, 1994–1998 CrossRef CAS PubMed.
- A. R. Yang, S. Lee, Y. D. Yoo, H. S. Kim, E. J. Jeong and J.-R. Rho, J. Nat. Prod., 2017, 80, 1688–1692 CrossRef CAS PubMed.
- S. Sato, F. Iwata, S. Yamada and M. Katayama, J. Nat. Prod., 2012, 75, 1974–1982 CrossRef CAS PubMed.
- L.-Y. Ma, W.-Z. Liu, L. Shen, Y.-L. Huang, X.-G. Rong, Y.-Y. Xu and X.-D. Gao, Tetrahedron, 2012, 68, 2276–2282 CrossRef CAS.
- X. Pan, D. Liu, J. Wang, X. Zhang, M. Yan, D. Zhang, J. Zhang and W. Liu, Cancer Sci., 2013, 104, 1476–1482 CrossRef CAS PubMed.
- M. P. Sobolevskaya, O. I. Zhuravleva, E. V. Leshchenko, S. S. Afiyatullov, Y. V. Khudyakova, N. Y. Kim, N. N. Kirichuk and S. A. Dyshlovoy, Chem. Nat. Compd., 2014, 50, 1122–1124 CrossRef CAS.
- W. Sun, X. Chen, Q. Tong, H. Zhu, Y. He, L. Lei, Y. Xue, G. Yao, Z. Luo, J. Wang, H. Li and Y. Zhang, Sci. Rep., 2016, 6, 26418 CrossRef CAS PubMed.
- H. Li, J. Jiang, Z. Liu, S. Lin, G. Xia, X. Xia, B. Ding, L. He, Y. Lu and Z. She, J. Nat. Prod., 2014, 77, 800–806 CrossRef CAS PubMed.
- W. Wang, B. Mun, Y. Lee, M. V. Reddy, Y. Park, J. Lee, H. Kim, D. Hahn, J. Chin, M. Ekins, S.-J. Nam and H. Kang, J. Nat. Prod., 2013, 76, 170–177 CrossRef CAS PubMed.
- Y. Lee, W. Wang, H. Kim, A. G. Giri, D. H. Won, D. Hahn, K. R. Baek, J. Lee, I. Yang, H. Choi, S.-J. Nam and H. Kang, Bioorg. Med. Chem. Lett., 2014, 24, 4095–4098 CrossRef CAS PubMed.
- L. Fremlin, M. Farrugia, A. M. Piggott, Z. Khalil, E. Lacey and R. J. Capon, Org. Biomol. Chem., 2011, 9, 1201–1211 CAS.
- T. Nogawa, S. Takahashi, Y. Sekiyama, H. Takagi, M. Uramoto, H. Koshino, M. Kawatani, T. Shimizu and H. Osada, J. Antibiot., 2013, 66, 247–250 CrossRef CAS PubMed.
- O. I. Zhuravleva, M. P. Sobolevskaya, S. S. Afiyatullov, N. N. Kirichuk, V. A. Denisenko, P. S. Dmitrenok, E. A. Yurchenko and S. A. Dyshlovoy, Mar. Drugs, 2014, 12, 5930–5943 CrossRef CAS PubMed.
- O. I. Zhuravleva, M. P. Sobolevskaya, V. A. Denisenko, N. N. Kirichuk, S. A. Dyshlovoy and S. S. Afiyatullov, Nat. Prod. Commun., 2016, 11, 207–210 Search PubMed.
- N. Bruns, W. Collisi, S. Bernecker, M. Stadler, C. Richter, H. Schwalbe and M. Kalesse, Eur. J. Org. Chem., 2015, 847–857 CrossRef CAS.
- T. Nogawa, S. Takahashi, A. Okano, M. Kawatani, M. Uramoto, T. Saito and H. Osada, J. Antibiot., 2012, 65, 123–128 CrossRef CAS PubMed.
- Y.-P. Chen, Q. Liu, H. Gao, H.-P. Lin, H.-Y. Tian, K. Hong, J. Li, R.-W. Jiang, X.-S. Yao and J.-S. Tang, RSC Adv., 2014, 4, 63324–63327 RSC.
- J. Huang, A.-L. Chen, H. Zhang, Z. Yu, M.-H. Li, N. Li, J.-T. Lin, H. Bai, J.-D. Wang and Y.-G. Zheng, Appl. Environ. Microbiol., 2015, 81, 5326–5334 CrossRef CAS PubMed.
- J. Li, L. Li, Y. Si, X. Jiang, L. Guo and Y. Che, Org. Lett., 2011, 13, 2670–2673 CrossRef CAS PubMed.
- K. Sadorn, S. Saepua, N. Boonyuen, P. Laksanacharoen, P. Rachtawee and P. Pittayakhajonwut, RSC Adv., 2016, 6, 94510–94523 RSC.
- M. S. Abdelfattah, K. Toume and M. Ishibashi, J. Antibiot., 2012, 65, 245–248 CrossRef CAS PubMed.
- R. M. Centko, S. Ramón-García, T. Taylor, B. O. Patrick, C. J. Thompson, V. P. Miao and R. J. Andersen, J. Nat. Prod., 2012, 75, 2178–2182 CrossRef CAS PubMed.
- G. Zhao, X. Li, W. Chen, Z. Xi and L. Sun, Fitoterapia, 2012, 83, 1590–1597 CrossRef CAS PubMed.
- J. R. Kesting, L. Olsen, D. Staerk, M. V. Tejesvi, K. R. Kini, H. S. Prakash and J. W. Jaroszewski, J. Nat. Prod., 2011, 74, 2206–2215 CrossRef CAS PubMed.
- P. Sun, Q. Zhao, F. Yu, H. Zhang, Z. Wu, Y. Wang, Y. Wang, Q. Zhang and W. Liu, J. Am. Chem. Soc., 2013, 135, 1540–1548 CrossRef CAS PubMed.
- P. Sun, Q. Zhao, Z. Wu, W. Zhang and W. Liu, J. Nat. Prod., 2015, 78, 301–305 CrossRef CAS PubMed.
- A. A. Singh, J. A. Rowley, B. D. Schwartz, W. Kitching and J. J. De Voss, J. Org. Chem., 2014, 79, 7799–7821 CrossRef CAS PubMed.
- S. Takahashi, A. Toyoda, Y. Sekiyama, H. Takagi, T. Nogawa, M. Uramoto, R. Suzuki, H. Koshino, T. Kumano, S. Panthee, T. Dairi, J. Ishikawa, H. Ikeda, Y. Sakaki and H. Osada, Nat. Chem. Biol., 2011, 7, 461–468 CrossRef CAS PubMed.
- C. Jiang, Z. Qi, Q. Kang, J. Liu, M. Jiang and L. Bai, Angew. Chem., Int. Ed., 2015, 54, 9097–9100 CrossRef CAS PubMed.
- R. Quach, D. F. Chorley and M. A. Brimble, Org. Biomol. Chem., 2014, 12, 7423–7432 CAS.
- H. Yao, J. Wang and R. Tong, Chem. Rec., 2017, 17, 1109–1123 CrossRef CAS PubMed.
- L. Song, H. Yao, Y. Dai, M. Wu and R. Tong, Tetrahedron Lett., 2016, 57, 4257–4263 CrossRef CAS.
- J. C. Green, G. L. Burnett IV and T. R. R. Pettus, Pure Appl. Chem., 2012, 84, 1621–1631 CrossRef CAS PubMed.
- I. Paterson, S. M. Dalby and P. Maltas, Isr. J. Chem., 2011, 51, 406–419 CrossRef CAS.
- J. S. Yadav, P. A. N. Reddy, Y. J. Reddy, S. Meraj and A. R. Prasad, Eur. J. Org. Chem., 2013, 6317–6324 CrossRef CAS.
- F.-M. Zhang, L. Peng, H. Li, A.-J. Ma, J.-B. Peng, J.-J. Guo, D. Yang, S.-H. Hou, Y.-Q. Tu and W. Kitching, Angew. Chem., Int. Ed., 2012, 51, 10846–10850 CrossRef CAS PubMed.
- L. Dong, V. A. Gordon, R. L. Grange, J. Johns, P. G. Parsons, A. Porzelle, P. Reddell, H. Schill and C. M. Williams, J. Am. Chem. Soc., 2008, 130, 15262–15263 CrossRef CAS PubMed.
- B. J. Albert, Y. Yamaoka and H. Yamamoto, Angew. Chem., Int. Ed., 2011, 50, 2610–2612 CrossRef CAS PubMed.
- W. Lee, S. Kang, B. Jung, H.-S. Lee and S. H. Kang, Chem. Commun., 2016, 52, 3536–3539 RSC.
- Y. Ogura, K. Ishigami and H. Watanabe, Tetrahedron, 2012, 68, 1723–1728 CrossRef CAS.
- L. C. Dias and E. C. de Lucca Jr, J. Org. Chem., 2017, 82, 3019–3045 CrossRef CAS PubMed.
- J. T. J. Spence and J. H. George, Org. Lett., 2015, 17, 5970–5973 CrossRef CAS PubMed.
- P. A. Hume, D. P. Furkert and M. A. Brimble, Org. Lett., 2013, 15, 4588–4591 CrossRef CAS PubMed.
- S. F. Seibert, A. Krick, E. Eguereva, S. Kehraus and G. M. König, Org. Lett., 2007, 9, 239–242 CrossRef CAS PubMed.
- S. Chang, S. Hur and R. Britton, Angew. Chem., Int. Ed., 2015, 54, 211–214 CrossRef CAS PubMed.
- D. V. Reddy, G. Sabitha, T. P. Rao and J. S. Yadav, Org. Lett., 2016, 18, 4202–4205 CrossRef CAS PubMed.
- J. E. Stok, C.-S. Lang, B. D. Schwartz, M. T. Fletcher, W. Kitching and J. J. De Voss, Org. Lett., 2001, 3, 397–400 CrossRef CAS PubMed.
- J. Y. Hamilton, S. L. Rössler and E. M. Carreira, J. Am. Chem. Soc., 2017, 139, 8082–8085 CrossRef CAS PubMed.
- D. C. Davis, K. L. Walker, C. Hu, R. N. Zare, R. M. Waymouth and M. Dai, J. Am. Chem. Soc., 2016, 138, 10693–10699 CrossRef CAS PubMed.
- S. Joung, R. Kim and H.-Y. Lee, Org. Lett., 2017, 19, 3903–3906 CrossRef CAS PubMed.
- Q. Xiao, W.-W. Ren, Z.-X. Chen, T.-W. Sun, Y. Li, Q.-D. Ye, J.-X. Gong, F.-K. Meng, L. You, Y.-F. Liu, M.-Z. Zhao, L.-M. Xu, Z.-H. Shan, Y. Shi, Y.-F. Tang, J.-H. Chen and Z. Yang, Angew. Chem., Int. Ed., 2011, 50, 7373–7377 CrossRef CAS PubMed.
- M. Wilsdorf and H.-U. Reissig, Angew. Chem., Int. Ed., 2012, 51, 9486–9488 CrossRef CAS PubMed.
- N. Yoneda, Y. Fukata, K. Asano and S. Matsubara, Angew. Chem., Int. Ed., 2015, 54, 15497–15500 CrossRef CAS PubMed.
- F. J. Fañanás, A. Mendoza, T. Arto, B. Temelli and F. Rodríguez, Angew. Chem., Int. Ed., 2012, 51, 4930–4933 CrossRef PubMed.
- X. Han and P. E. Floreancig, Angew. Chem., Int. Ed., 2014, 53, 11075–11078 CrossRef CAS PubMed.
- M. F. Elsebai, M. Nazir, S. Kehraus, E. Egereva, K. N. Ioset, L. Marcourt, D. Jeannerat, M. Gütschow, J.-L. Wolfender and G. M. König, Eur. J. Org. Chem., 2012, 6197–6203 CrossRef CAS.
- Z. Ren, Y. Hao and X. Hu, Org. Lett., 2016, 18, 4958–4961 CrossRef CAS PubMed.
- J. Huang, J. R. Yang, J. Zhang and J. Yang, J. Am. Chem. Soc., 2012, 134, 8806–8809 CrossRef CAS PubMed.
- M. Xuan, I. Paterson and S. M. Dalby, Org. Lett., 2012, 14, 5492–5495 CrossRef CAS PubMed.
- H. Cheng, Z. Zhang, H. Yao, W. Zhang, J. Yu and R. Tong, Angew. Chem., Int. Ed., 2017, 56, 9096–9100 CrossRef CAS PubMed.
- L. Tomas, G. Boije af Gennäs, M. A. Hiebel, P. Hampson, D. Gueyrard, B. Pelotier, J. Yli-Kauhaluoma, O. Piva, J. M. Lord and P. G. Goekjian, Chem.–Eur. J., 2012, 18, 7452–7466 CrossRef CAS PubMed.
- H. Fuwa, K. Sekine and M. Sasaki, Org. Lett., 2013, 15, 3970–3973 CrossRef CAS PubMed.
- H. Fuwa, T. Muto, K. Sekine and M. Sasaki, Chem.–Eur. J., 2014, 20, 1848–1860 CrossRef CAS PubMed.
- G. V. M. Sharma, G. Srikanth and P. P. Reddy, Org. Biomol. Chem., 2012, 10, 8119–8124 CAS.
- Y. Zhang, Q. Ye, X. Wang, Q.-B. She and J. S. Thorson, Angew. Chem., Int. Ed., 2015, 54, 11219–11222 CrossRef CAS PubMed.
- A. Yamamoto, A. Ueda, P. Brémond, P. S. Tiseni and Y. Kishi, J. Am. Chem. Soc., 2012, 134, 893–896 CrossRef CAS PubMed.
- S. Badrinarayanan, C. J. Squire, J. Sperry and M. A. Brimble, Org. Lett., 2017, 19, 3414–3417 CrossRef CAS PubMed.
- P. Pal and S. Nanda, Org. Lett., 2017, 19, 1164–1167 CrossRef CAS PubMed.
- J. Lehmann, J. Richers, A. Pöthig and S. A. Sieber, Chem. Commun., 2017, 53, 107–110 RSC.
- S. Newton, C. F. Carter, C. M. Pearson, L. de, C. Alves, H. Lange, P. Thansandote and S. V. Ley, Angew. Chem., Int. Ed., 2014, 53, 4915–4920 CrossRef CAS PubMed.
- M. Scheepstra, S. A. Andrei, M. Y. Unver, A. K. H. Hirsch, S. Leysen, C. Ottmann, L. Brunsveld and L.-G. Milroy, Angew. Chem., Int. Ed., 2017, 56, 5480–5484 CrossRef CAS PubMed.
- B. Borgström, X. Huang, C. Hegardt, S. Oredsson and D. Strand, Chem.–Eur. J., 2017, 23, 2077–2083 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |