Microwave-assisted fast synthesis of hierarchical NiCo2O4 nanoflower-like supported Ni(OH)2 nanoparticles with an enhanced electrocatalytic activity towards methanol oxidation†
Bingrong
Wang
ab,
Yang
Cao
a,
Yong
Chen
a,
Ruzhi
Wang
 b,
Xiaohong
Wang
a,
Xiaoyong
Lai
b,
Xiaohong
Wang
a,
Xiaoyong
Lai
 c,
Chunhui
Xiao
c,
Chunhui
Xiao
 d,
Jinchun
Tu
d,
Jinchun
Tu
 *a and
Shujiang
Ding
*a and
Shujiang
Ding
 *d
*d
aState Key Laboratory of Marine Resource Utilization in South China Sea, Key Laboratory of Tropical Biological Resources of Ministry of Education Hainan University, Haikou 570228, P. R. China. E-mail: tujinchun@hainu.edu.cn; Fax: +86 898 66259764; Tel: +86 898 66259764
bCollege of Materials Science and Engineering, Beijing University of Technology, Beijing 100124, P. R. China
cState Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering and College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, P. R. China
dDepartment of Applied Chemistry, School of Science, MOE Key Laboratory for Nonequilibrium Synthesis and Modulation of Condensed Matter, State Key Laboratory for Mechanical Behavior of Materials, Xi'an Jiaotong University, Xi'an 710049, P. R. China. E-mail: dingsj@mail.xjtu.edu.cn; Fax: +86 029 82668391; Tel: +86 029 82668391
First published on 10th November 2017
Abstract
NiCo2O4 nanoflowers were synthesized through a microwave-assisted hydrothermal method and used as a type support for Ni(OH)2 nanoparticles. In the three-dimensional NiCo2O4 nanoflowers, two-dimensional ultrathin nanosheets supported the Ni(OH)2 nanoparticles by homogeneous precipitation. The materials were characterized by field-emission scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and Raman spectroscopy. The electrochemical oxidation of methanol was probed through the NiCo2O4/Ni(OH)2 modification on a glassy carbon electrode in an alkaline medium by employing cyclic voltammetry (CV) and chronoamperometry (CA). The current density of the NiCo2O4/Ni(OH)2 electrode in 1 M KOH with 0.5 M methanol ascends to 92.3 A g−1 and restores to 94.6% of the primitive value through the replacement with a new solution after a long-term CV cycling (500 cycles). Therefore, the compounds further corroborate their excellent electrocatalytic activity and superb perennial stability for methanol oxidation. This study demonstrates that NiCo2O4/Ni(OH)2 is a peculiar material with an outstanding performance in direct methanol fuel cells.
1. Introduction
In recent years, fuel cells became the prospective driving engine of green power for future energy conversion devices.1 Among them, direct methanol fuel cells (DMFCs) have attracted significant attention because of their high-efficiency energy conversion, easy refueling, low operating temperature, and environmental friendliness.2,3 Noble metals such as Pt and Pd have been widely applied and extensively recognized as the most effective electrocatalysts in DMFCs.4–8 However, the inherent drawbacks of Pt and Pt-based electrocatalysts,9,10 such as poor abundance, high cost, susceptibility to deactivation, easy degradation, and CO poisoning, restrict their practical applications. Thus, it is necessary to find a substitute for Pt in Pt-based catalysts in DMFCs. It is well-acknowledged that the expensive input into the methanol electro-oxidation catalysts is a primary obstacle for their extensive application. Therefore, the development of non-Pt and non-Pd nanocatalysts with a high catalytic activity is vital. Tremendous efforts have been made to explore the availability of lower-cost materials as promising alternatives, which are suitable for the fabrication of excellent electrochemical sensors.In the last few years, many researchers have focused on optimizing the design of catalysts and enhancing their catalytic efficiency, including Pt alloys that contain a secondary metal, such as Sn,11 Cu,12 Pd,13 and Au.14 In addition, the catalyst research has recently been concentrated on some metal oxides, for instance, SnO2,15 CeO2,16 Co3O4,17 Fe3O4,18 Cr2O3,19 NiO,20 and MnOx,21 which can also act as potential candidates that improve the poison tolerance, suppress the surface oxidation of catalysts, and enhance the electrocatalytic performance of the electrodes. However, their poor catalytic activity and low conductivity have limited their application. NiCo2O4, derived from the incorporation of Ni atoms into Co3O4, has a better catalytic activity than that of Co3O4 alone in terms of the enhanced electrical conductivity and availability of numerous catalytically active sites.22,23 Furthermore, NiCo2O4 is particularly promising because its methanol catalytic activities could be further improved by the fast electron transfer from the substituted Ni3+ to Co3+ in the spinel lattice of NiCo2O4.24 However, the high electronic conductivity of NiCo2O4 is a significant issue for further increasing of the electrochemical activities probably because of the simultaneous existence of the redox reactions of cobalt and nickel ions. Although the better activity has been achieved by the electrocatalyst, further enhancements in its performance are possible by either modifying the synthesis method or tailoring the NiCo2O4 morphology. In comparison with the well-established synthetic approaches such as hydrothermal,25,26 sol–gel,27 and electro-deposition,28 the microwave-assisted hydrothermal methods have significant advantages from a kinetic perspective, being fast, simple, environmental friendly, easy-to-operate, less expensive, and periodically short.29,30 Meanwhile, microwave hydrothermal methods had influence the highly defective of NiCo2O4 in progress and speed of nanomaterial synthesis due to the presence and concentration of defects. Based on the above discussion, vast lattice mismatch and lattice edges within the nanosheet in the ultrafast reaction rate are produced.31
The catalytic performance is greatly impacted by the morphology of the material. Its hierarchical structure with a branched architecture can provide some intrinsic surface properties, including high surface roughness, large surface-to-volume ratio, multiple high angle edges, and sharp tips. Hence, multifarious hierarchical nanostructures, such as wires,32 plates,33 rods,34 spheres,35 and flowers,24 have been studied and reported. Among them, the flower nanostructure is considered as an effective high-activity two-dimensional (2D) structure36,37 on account of its better utilization efficiency, fantastic catalytic performance, high surface area, and lower density. The differences with its solid counterparts and defective nanosheets are the abundant active edge sites and surface defects.
In the electrocatalytic materials, the active sites are often divided into macro-, micro-, and mesopores. Among them, the macro-pores promote the facile diffusion of species towards the active sites and also away from them.38 Moreover, it is well-known that the three pore sizes mentioned above would provide short electron and ion transport paths and large surface areas, resulting in an enhanced catalytic activity.39,40 In this study, we reserve those characteristics of NiCo2O4 by using the microwave-assisted synthesis. Moreover, it is significant to introduce a second phase to enhance the catalytic properties of the material because the catalytic performance of a single NiCo2O4 is limited in comparison with that of other precious metals. Therefore, the interface engineering is utilized to build the structural integrity of the electrode materials to improve its electrochemical property.41,42 The presence of an interface between two material layers exhibits a charge discontinuity and a built-in electric field, which imposes significant effects on the charge flow and the surface faradaic (redox) reactions.43 It is necessary to introduce the second phase to build interface engineering to improve the catalytic performance of the material.
Nickel hydroxide, Ni(OH)2, is an active transition metal hydroxide employed as a catalytic material for methanol oxidation. The use of Ni(OH)2 as electrodes is popular for its enhancement the surface redox reaction, excellent electrochemical performance, easy preparation, natural abundance, and environmental compatibility.44 Small Ni(OH)2 particles can also hasten the redox reaction and shorten the diffusion path.45,46 However, the aggregation of nanoscale material is inevitable owing to the existing high surface energy, which can affect the catalytic behavior. To overcome the poor utilization of Ni(OH)2 nanoparticles due to its aggregation, a conductive substrate is used to serve as the supports for the dispersion of the nanocatalyst. Therefore, NiCo2O4 was reliably selected because it can function as both a base material and a catalyst.
In this study, a facile and efficient strategy in the synthesis of the hierarchically structured NiCo2O4 supported Ni(OH)2 nanoparticles is reported. First, a simple and easy microwave-assisted method to synthesize the flower-like precursors was adopted, where the flower-shaped precursors were easily converted into porous ultrathin flower-like spinel NiCo2O4 with a well-conserved morphology of small nanoparticles. Second, Ni(OH)2 nanoparticles deposit on NiCo2O4 through homogeneous precipitation while maintaining the original NiCo2O4 morphology. Finally, the as-prepared NiCo2O4/Ni(OH)2 nanocomposites were successfully applied in the electrocatalytic oxidation of methanol, exhibiting a high electrocatalytic activity and a superb long-term stability. Above all, this strategy is of practical interest for the development of low-cost and high-performance electrocatalysts.
2. Experimental
2.1 Chemicals
Ni(NO3)2·6H2O and Co(NO3)2·6H2O were obtained from Sinopharm Chemical Reagent Co., Ltd. Concentrated ammonia, methanol, and absolute ethanol were provided by Xilong Scientific Co., Ltd. Hexamethylenetetramine (HMT) was acquired from Aladdin Industrial Corporation. All materials were used without any further purification.2.2 Apparatus
The crystal structures of NiCo2O4 and NiCo2O4/Ni(OH)2 were characterized using a D8 Tools X-ray diffraction (XRD) instrument at 40 kV and 30 mA with a Cu-Kα radiation (λ = 1.5406 Å). The structure of the NiCo2O4/Ni(OH)2 nanocomposites was confirmed by X-ray photoelectron spectroscopy (XPS) and Reflex Laser Raman spectrograph (Renishaw Corporation). The morphologies of NiCo2(OH)x, NiCo2O4, and NiCo2O4/Ni(OH)2 were observed on a MIRA3 TESCAN field-emission scanning electron microscopy (FESEM) and JEOL-2100F transmission electron microscopy (TEM).The electrochemical experiments by cyclic voltammetry (CV) and chronoamperometry (CA) were performed using a SP-200 Electrochemical Workstation (Bio-Logic Science Instruments). The modified glass carbon electrode (GCE), a Pt wire, and an Ag/AgCl (saturated solution) electrode were served as working, auxiliary, and reference electrodes, respectively.
2.3 Experimental section
2.4 Electrode modification
Prior to the electrode modification, the GCE was carefully polished with 0.05 μm alumina powder and sonicated in DI water and ethanol for 6 min to obtain a smooth surface. The NiCo2O4/Ni(OH)2 aqueous solution was added dropwise to the GCE surface and the final product was air-dried for further use.3. Results and discussion
3.1 Characterization of NiCo2O4/Ni(OH)2
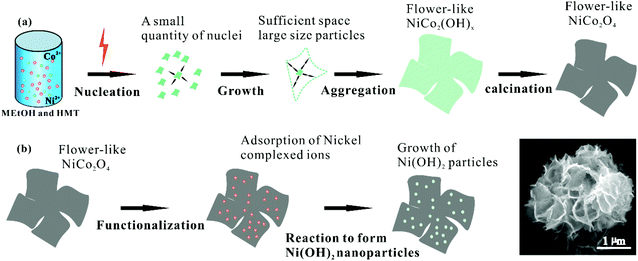
On the basis of the experimental observation and literature research, there is a mechanism for the morphogenesis evolution process, in which a number of factors affect the formation of a specific structure, such as van der Waals forces, hydrogen bonding, intrinsic crystal contraction, electrostatic and dipolar fields, and crystal-face attraction.24Scheme 1 shows the synthetic process to obtain flower-like NiCo2O4 and the growth process to achieve NiCo2O4 decorated with Ni(OH)2 nanoparticles. As shown in Scheme 1(a), the OH− ions are gradually released by HMT hydrolysis at high temperature, which facilitates the yield of the crystal nucleus of NiCo2(OH)x in the initial reaction stage under microwave irradiation. Then, the freshly formed NiCo2(OH)x nuclei aggregate due to their thermodynamic instability with high surface energy. As the reaction proceeds, the concentration of the reactants decreases and some active sites on the surface of the initially formed precursor aggregate. New particles continuously deposit on the previously formed particles, increasing the areas of the agglomerates and leading to the formation of the small petal-like structures under the influence of HMT, which further grow along the specific directions to form a uniform flower-like NiCo2(OH)x. After calcination, NiCo2(OH)x is transformed to porous ultrathin flower-like spinel NiCo2O4 with a well-conserved morphology of some small nanoparticles. As shown in Scheme 1(b), we chose ammonia as a low-cost coordination agent in decorating the NiCo2O4 surface and added the former to the Ni(NO3)2 solution. When the ammonia concentration is relatively low, the ionic product of Ni(OH)2 exceeds the solubility product and the solution becomes turbid (1). Raising the amount of concentrated ammonia decreases the concentration of free Ni2+ and produces more Ni(NH3)62+, which manifests that Ni(OH)2 solution becomes transparent and clear (2). When Ni(NH3)62+ is reacted with NiCo2O4, the former adsorbs on the surface of the latter through van der Waals, cohesive, or other attractive forces; the evolution of gaseous ammonia from Ni(NH3)62+ by heating increases the concentration of free Ni2+(3). When Ni2+ reaches a certain degree of solubility, Ni(OH)2 nanoparticles simultaneously form in the solution (4). The SEM images show the corresponding morphology of the samples.| Ni(NO3)2 + 2NH4OH → Ni(OH)2↓ + 2NH4+ + NO3− | (1) |
| Ni(OH)2 + 6NH4+ → Ni(NH3)62+ + 2H2O + 2H+ | (2) |
| Ni(NH3)62+ → Ni2+ + 6NH3↑ | (3) |
| Ni2+ + 2OH− → Ni(OH)2↓ | (4) |
The FESEM images shown in Fig. 1 display the morphologies and microstructures of the synthetic precursors and NiCo2O4/Ni(OH)2. The NiCo2(OH)x precursor exhibits a solid nanoflower-like structure with a diameter of approximately 2 μm as illustrated in Fig. 1(a). Fig. 1(b) presents the FESEM image of the integral NiCo2O4 nanoflowers obtained after the calcination of NiCo2(OH)x in air. Although the calcination process (300 °C for 240 min) causes a slight destruction of the nanosheets, NiCo2O4 maintains its nanoflower structure. This unique structure endows a large specific surface area (SSA) of NiCo2O4 and this intertwined feature also facilitates the electrolyte penetration into NiCo2O4 nanosheets for the charge transfer at the electrolyte–electrode interface. Fig. 1(c and d) displays the images of NiCo2O4/Ni(OH)2 at different magnifications, where NiCo2O4/Ni(OH)2 composite maintains the NiCo2O4 morphology with a nanosheet thickness of roughly 20 nm. However, the surface of NiCo2O4/Ni(OH)2 composite becomes rougher and contains numerous Ni(OH)2 nanoparticles that provide more active sites. To further investigate the spatial distribution and proportion of the elements, energy dispersive X-ray spectrometry (EDS) mapping analysis was carried out. As shown in Fig. S1(a),† there are large areas of NiCo2O4/Ni(OH)2 nanoflowers with uniform size. Fig. S1(b–e)† displays the spatial distributions of the elements across the NiCo2O4/Ni(OH)2 nanocomposites, which affirm the presence of Ni, Co, and O atoms. As presented in Fig. S1(f),† the corresponding EDS results for these NiCo2O4/Ni(OH)2 nanocomposites reveal that the Ni/Co atomic ratios is 0.67, which is higher than that for NiCo2O4 (0.5), indicating the presence of Ni(OH)2. The existence of Ni(OH)2 nanoparticles can also be testified through XPS and Raman spectroscopy.
 | ||
| Fig. 1 FESEM images of (a) NiCo2(OH)x; (b) NiCo2O4; and (c, d) NiCo2O4/Ni(OH)2 at different magnifications. | ||
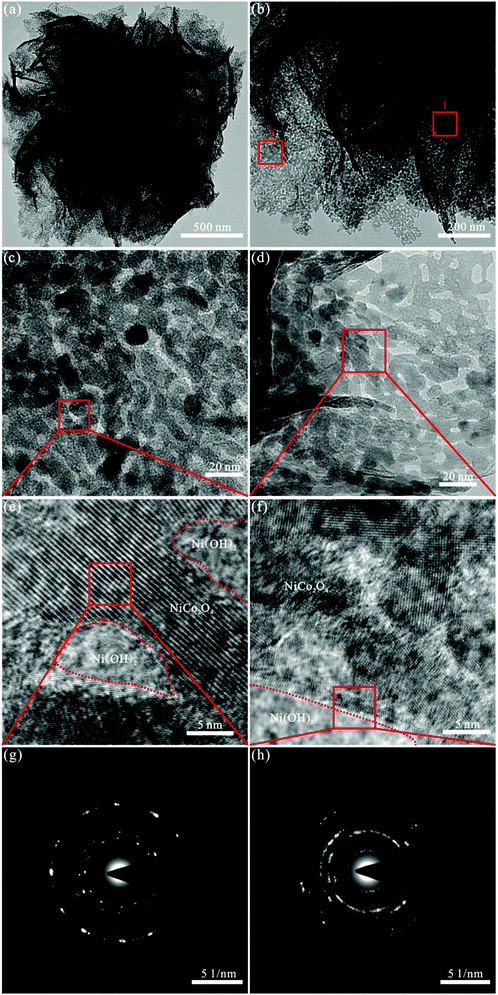
Fig. 2 presents the morphological and structural features of the NiCo2O4/Ni(OH)2 hybrids obtained through TEM and SAED. Fig. 2(a and b) displays the TEM images of the NiCo2O4/Ni(OH)2 hybrids, exhibiting flower-like morphologies at different magnifications. The diameter of NiCo2O4/Ni(OH)2 is about 2 μm and the thickness of nanosheet is roughly 20 nm, which is in accordance with those obtained from the SEM results. Fig. 2(c) exhibits the high-resolution TEM image of the selected square area 1 in Fig. 2(b). It can be seen that NiCo2O4 consists of small particles with the diameter of around 15 nm and more secondary pores. Fig. 2(d) exhibits the high-resolution TEM image of the selected square area 2 in Fig. 2(b) and reveals that several small Ni(OH)2 nanoparticles adhere to the NiCo2O4 edges with a diameter of approximately 10 nm and many secondary pores, confirming the hybrid nanostructure morphology of NiCo2O4/Ni(OH)2. The high-resolution TEM (HRTEM) images of the selected square areas in Fig. 2(c and d), shown in Fig. 2(e and f), clearly disclose that there are lattice edges and surface defects in high density, and Ni(OH)2 is present in the secondary pores and edges of NiCo2O4, indicating the creation of more surface and additional active edge sites. As shown in Fig. 2(g), the samples are in the form of visible spots and well-defined rings, implying the polycrystalline nature of NiCo2O4. The spots become less visible in Fig. 2(h), suggesting that the crystallinity decreases due to the presence of Ni(OH)2 and the charge transfer between NiCo2O4 and Ni(OH)2. Thus, the NiCo2O4/Ni(OH)2 nanoflowers are in low crystallinity and its polycrystalline characteristics are coherent with the XRD results.
The XPS was implemented to obtain further information on the elemental composition, oxidation state, and the surface chemistry of NiCo2O4/Ni(OH)2. From the results shown in Fig. 3(a), the survey spectrum reveals the presence of Ni, Co, and O atoms. The binding energies for C 1s, Si 2p, and Al 2p were 300 eV, 103.1 eV, and 74.9 eV, respectively.49,50 As shown in Fig. 3(b), using the Gaussian fitting method, two spin–orbit peaks at about 855.75 and 873.65 eV in the Ni 2p spectrum, which correspond to the Ni 2p3/2 and Ni 2p1/2 electronic configurations, respectively, are obtained, confirming the characteristics of Ni3+ and Ni2+, respectively. The other two small bulges with the peaks at approximately 881.3 eV and 862.7 eV are defined as satellite peaks. The polyvalent interactions in the compounds allow for rapid charge transport through the electrode interface. According to the Gaussian–Lorentzian curve in the Co 2p spectrum in Fig. 3(c), two strong peaks at about 780.4 eV and 794.95 eV are assigned to the Co 2p3/2 and Co 2p1/2 electronic configurations, which fit the two spin–orbit peaks that correspond to Co2+ and Co3+, respectively.51 The other two peaks are shake-up satellites (sat.).52 The NiCo2O4/Ni(OH)2 composite containing the Co3+/2+ and Ni3+/2+ couples results in the synergistic contribution of both metal ions to the electrochemical processes. Fig. 3(d) shows the O 1s XPS profile of NiCo2O4/Ni(OH)2, in which the broad peak at about 530 eV can be deconvoluted into four peaks. The peak at 529.57 eV represents the typical metal–oxygen bonds of O–M (M = Ni/Co). The peak appearing at 530.7 eV corresponds to Ni–OH. The peaks at 531.56 eV and 532.8 eV can be assigned to the oxygen ions in low-coordination sites at the surface and the multiple of physi- and chemisorbed water molecules at or within the surface, respectively.
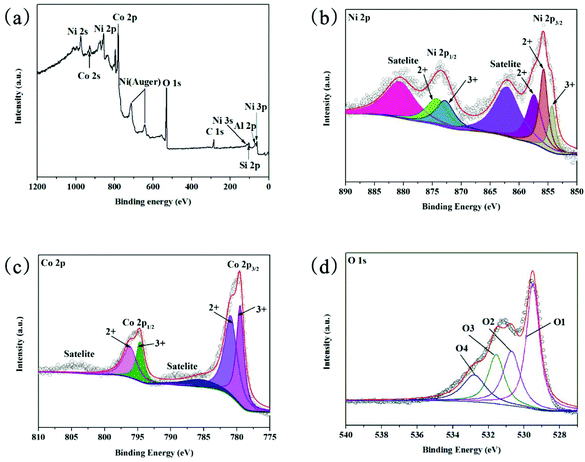 | ||
| Fig. 3 The XPS spectra of the NiCo2O4/Ni(OH)2 hybrid nanostructure. (a) Survey spectrum, (b) Ni 2p, (c) Co 2p, and (d) O 1s. | ||
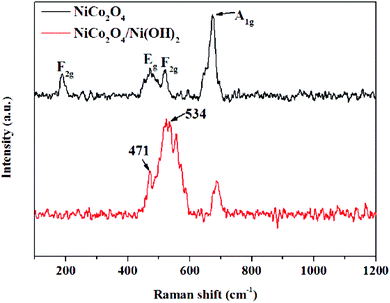
Raman spectroscopy was implemented to prove the manifestation of Ni(OH)2 and the synergy of Co3+/2+ and Ni3+/2+ ions (Fig. 4). The peaks at 187, 473, 521, and 673 cm−1 correspond to F2g, Eg, F2g, and A1g modes of the NiCo2O4 nanoflowers, respectively.53 The peak at 471 cm−1 is also observable in the NiCo2O4/Ni(OH)2 nanocomposite, which arises from the symmetrical Ni–OH stretching along the longitudinal optic. A new peak at 534 cm−1 can also be observed, which indicates the stretching/vibration of Ni–O;54,55 the other peaks correspond to NiCo2O4 and Ni(OH)2. These results demonstrate that Ni(OH)2 nanoparticles are supported on the surface of NiCo2O4.
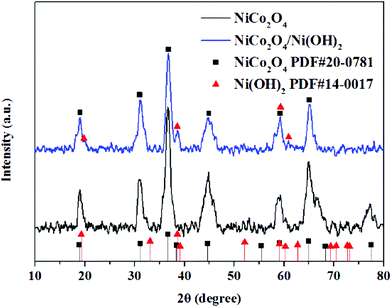
The metal oxides and metal oxide-based hybrids in this study were subjected to XRD for investigating their purity and phases. The black peaks of NiCo2O4 at 19°, 31.3°, 36.6°, 44.9°, 59.3°, 65.1°, and 77.4° correspond to the (111), (220), (311), (400), (511), (440), and (533) planes, indicating the cubic crystal structure of NiCo2O4 (JCPDS card no. 20-0781).56 The absence of the impurity peaks illustrates that the fabricated nanomaterial is of high purity and crystallinity. Moreover, the blue curve in Fig. 5 displays the peaks located at similar positions to those of the peaks of cubic NiCo2O4 with the presence of a few small peaks, corroborating the crystal structure of Ni(OH)2 (JCPDS card no. 14-0017). However, the characteristic peaks of Ni(OH)2 are not distinctly observed because the content of Ni(OH)2 is lower than that of NiCo2O4 and is uniformly distributed on the NiCo2O4 surface. Moreover, because no other peaks were detected, the fabricated product is high purity.
3.2 Electrocatalytic oxidation of methanol at the NiCo2O4/Ni(OH)2 modified electrode
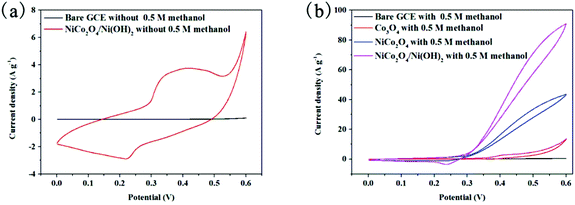
To investigate the electrocatalytic performance of NiCo2O4/Ni(OH)2, the direct modification of GCE toward methanol oxidation (MOR) was performed. For comparison, MORs in the presence of GCE, Co3O4/GCE, NiCo2O4/GCE, and NiCo2O4/Ni(OH)2/GCE were investigated under the same conditions. Fig. 6(a) shows the CV curves of NiCo2O4/Ni(OH)2/GCE and GCE in 1 M KOH in the absence of 0.5 M methanol electrolytes from 0 to 0.6 V at a scan rate of 10 mV s−1. For the NiCo2O4/Ni(OH)2 electrode, there is an oxidation peak at ∼0.4 V, appearing in the anodic peak current, and a reduction peak at ∼0.22 V during the reverse scan, indicating that the redox reaction of NiCo2O4/Ni(OH)2 occurs in an alkaline environment, which is beneficial to methanol oxidation.57 The redox reactions in the alkaline electrolyte can be explained as follows (5)–(7):30,58–60| NiCo2O4 + OH− + H2O ↔ NiOOH + 2CoOOH + 2e− | (5) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (6) |
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e− | (7) |
As shown in Fig. 6(b), after the addition of 0.5 M methanol into 1 M KOH, the current density sharply increases at 0.3 V because of the methanol electro-oxidation by the NiCo2O4/Ni(OH)2 electrode. The methanol electro-oxidation on the NiCo2O4/Ni(OH)2 electrode can be simply expressed by eqn (8):61–63
| \eqalign{2MOOH + 2CH3OH + 5/2O2 → \tab2M(OH)2 + 2CO2 + 3H2O \cr \tab(M = Ni, Co) | (8) |
The current density in 1 M KOH/0.5 M methanol is 92.3 A g−1, suggesting that the NiCo2O4/Ni(OH)2 electrode exhibits a high activity for methanol, which may result from the influence of a large SSA in GCE. The electrocatalytic activities of NiCo2O4/Ni(OH)2/GCE towards methanol oxidation are examined. From the black and blue curves in Fig. 6(a), NiCo2O4/Ni(OH)2/GCE and GCE manifest distinctly different behaviors in 1 M KOH in the absence of 0.5 M methanol. GCE has a much lower current than that of NiCo2O4/Ni(OH)2/GCE, demonstrating that NiCo2O4/Ni(OH)2 is important in the electro-catalytic oxidation of methanol from the reverse scan.
To further explain that NiCo2O4/Ni(OH)2 can be a favorable catalyst for methanol oxidation, the electrocatalytic activity of GCE, Co3O4/GCE, NiCo2O4/GCE, and NiCo2O4/Ni(OH)2/GCE in 1 M KOH/0.5 M methanol at a scan rate of 10 mV s−1 were performed and presented in Fig. 6(b) for comparison. Compared to other electrodes, NiCo2O4/Ni(OH)2/GCE exhibits a large increase in the current density, which arises from the synergistic effects of both nickel and cobalt ions and not from monometallic Co3O4, the strong faradaic redox reaction, or the shorter diffusion path of polycrystalline Ni(OH)2 nanoparticles. This result should be ascribed to the presence of more defects and active sites on NiCo2O4/Ni(OH)2. Furthermore, the onset potential towards methanol oxidation of NiCo2O4/Ni(OH)2 is approximately 0.3 V, which is much lower than that of the others during the direct electro-oxidation of methanol. It is beneficial to refrain an electronic effect or a structural change when nickel and cobalt act as chemical modifiers. Second, the addition of nickel promotes the electron transfer and contributes in achieving a higher oxidation state during methanol oxidation. Table 1 lists the anodic peak potentials of different Ni- and Co-based electrocatalysts from the previous studies and compares them with that of the present study. The outstanding performance of the NiCo2O4/Ni(OH)2-modified electrode is on account of the facile internal electron transitions between Co 3d-t2g to Co 3d-eg (or from Ni 3d-t2g to Ni 3d-eg) in the electrocatalytic process.
| Electrode material | Anodic peak potential (V) | Peak current density (mA cm−2) | Methanol concentration (M) | Ref. |
|---|---|---|---|---|
| NiCo2O4-RGO | 0.6 | 0.05 | 0.5 | 64 |
| NiCo2O4 spinel nanoparticles | 0.6 | 93 | 0.5 | 26 |
| NiCo2O4/Ni foam nanosheet | 0.6 | 111 | 0.5 | 63 |
| Porous NiCo2O4 | 0.6 | 125 | 0.5 | 65 |
| Ni–Co alloy | 0.6 | 2.3 | 0.5 | 66 |
| NiCo2O4/Ni(OH)2 | 0.6 | 132 | 0.5 | This work |
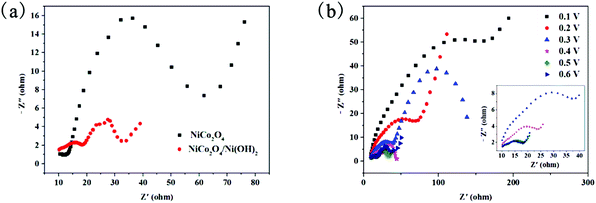
To prove that the addition of Ni(OH)2 accelerates the electron or charge transport in fuel cell reactions, the electrochemical impedance spectroscopy (EIS) was conducted. Moreover, EIS is convenient for the investigation of methanol oxidation of NiCo2O4/Ni(OH)2/GCE. Fig. 7(a) shows the Nyquist diagrams of NiCo2O4/GCE and NiCo2O4/Ni(OH)2/GCE recorded at 0.5 V vs. SCE in 1 M KOH with 0.5 M methanol. In principle, the EIS plots contain two parts: a high-frequency semicircle related to a charge transfer and a low-frequency domain related to a diffusion process. The impedance spectrum of NiCo2O4/GCE consists of the high-frequency semicircle and the low-frequency straight line. In contrast, the impedance spectrum of NiCo2O4/Ni(OH)2/GCE consists of two slightly depressed semicircles at high frequency and a straight line at low frequency. Two slightly depressed semicircles in the impedance spectrum indicate that the spectrum possesses two time constants; from the straight line at low frequency, it can be concluded that this spectrum has Warburg impedance. The two time constants originate from two separated relaxation processes. The relaxation process occurs when the state variable deviates from the steady-state value after being disturbed and when the disturbance disappears, the state variable returns to the original steady-state value. The depressed semicircle in the high-frequency region can be related to the combination of the charge transfer resistance and the double layer capacitance. The subsequent semicircle was related to the adsorption of the reaction intermediate on the electrode surface. These two different processes are displayed as the two semicircles in the impedance diagram. In the methanol oxidation reaction, CH3OH produces the intermediates after the complex procedures of the adsorption and several dehydrogenations on NiCo2O4/Ni(OH)2/GCE. It is likely that more than one adsorbed intermediate is present, such as COads, CH2O−, and CH3O−. However, among those intermediates, COads is considered as the primary carbonaceous adsorbate on the catalyst surface during methanol oxidation.67–69 It is plausible that only one adsorbed intermediate has significant stability to be detected by EIS, but this does not exclude the possibility of other parallel pathway(s) or multiple product formation. The low-frequency straight line is attributed to the Warburg impedance, which is caused by the diffusion/transport process on the electrode surface. A key point in this Nyquist diagram is that the diameter of the semicircle at the high-frequency region of NiCo2O4/Ni(OH)2/GCE is much smaller than that of NiCo2O4/GCE. Hence, NiCo2O4/Ni(OH)2/GCE has a higher charge transfer rate than that of NiCo2O4/GCE. Another key finding is that the emergence of an inductive loop indicates the improved adsorption of the reaction intermediate on the electrode surface.
Fig. 7b shows the EIS plots of NiCo2O4/Ni(OH)2/GCE recorded at different potentials in 1 M KOH/0.5 M methanol. When the onset potentials are 0.1 and 0.2 V, only one capacitive semicircle at the high-frequency region is visible and the low-frequency response becomes inductive as the potential increases, approaches or exceeds 0.3 V. As the potential increases, the “diameter” of both capacitive and inductive loops decreases (even though these two are not perfect semicircles, the diameter will be used here for ease of comprehension). However, the charge transfer resistances at 0.5 and 0.6 V are almost equal and the low-frequency inductive loop at 0.6 V is larger than that at 0.5 V. To elucidate the above phenomenon, we made the following assumptions:
1. A decrease in the charge transfer resistance occurs in the tested potential range (0.1 and 0.2 V before the onset). This phenomenon can be attributed to the large coverage of methanol residues with the increase in potential. If the removal of the residues does not occur or the removal is slower than the formation, the charge transfer resistance decreases with increase in potential.
2. After the onset potential, the methanol oxidation current starts increasing with increase in potential (Fig. 6(a) CV) and an inductive behavior appears in the Nyquist plot (Fig. 7(b)). These phenomena indicate a certain change in the reaction mechanism below and above 0.3 V. This inductive behavior can now be rationalized by postulating the slowness of the adsorbed intermediate coverage relaxation: the adsorbed intermediate coverage decreases with increasing the potential, but this process takes some time after a potential perturbation and before establishing new steady-state coverage. This phase-delay leads to the observed inductive behavior of the impedance. Considering the complexity of the methanol electro-oxidation reaction, it is likely to have more than one adsorbed intermediate.
3. The charge transfer resistances at 0.5 and 0.6 V are almost equal and the low-frequency inductive loop at 0.6 V is larger than that at 0.5 V. When the potential exceeds 0.5 V, the material limits the further oxidation of the adsorbed intermediate, which is slower than the adsorption and dehydrogenation of methanol. Therefore, the charge transfer resistance no longer decreases with increase in potential. Moreover, the surface of NiCo2O4/Ni(OH)2/GCE adsorbs numerous aggregates of the intermediate because of the high potential, which leads to the increase of the inductor semicircles.
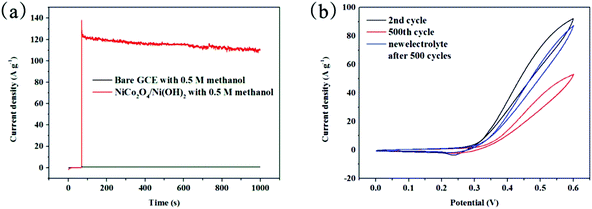
CA is a useful tool for investigating the electrochemical stability of the electrocatalysts. According to the discussion above, 0.6 V was selected as the optimal potential for the CA tests and they were performed for 1070 s. Fig. 8(a) depicts the stability of GCE and NiCo2O4/Ni(OH)2/GCE during methanol oxidation, which does not show any distinct decay in 1000 s. A slight current variation for NiCo2O4/Ni(OH)2/GCE exists during the first several seconds as a result of poisoning of the oxidation intermediate. Nevertheless, NiCo2O4/Ni(OH)2/GCE does not exhibit any notable decay after 1000 s, demonstrating the superb stability of the electrode in methanol oxidation and its application as an ideal supporter in DMFCs. Moreover, NiCo2O4/Ni(OH)2/GCE has a higher current density than that of GCE, which manifests that the former exhibits a higher electrocatalytic activity towards methanol oxidation and the effect of the latter is too negligible to quantify. The results from CA are in good agreement with the data obtained from CV (Fig. 6(a)).
The current density of NiCo2O4/Ni(OH)2/GCE was lower because of the amount of methanol reduced. Long-stability is another factor to assess the performance of the catalyst during methanol oxidation.70 CV was used to investigate the electrode stability. The CV curves in Fig. 8(b) show that the current density of NiCo2O4/Ni(OH)2/GCE retains 57.5% of its original value after 500 cycles at a scanning rate of 10 mV s−1. Moreover, the current density of NiCo2O4/Ni(OH)2/GCE at 0.6 V can return to 94.6% of the original value by replacing with a new homogeneous solution. Therefore, NiCo2O4/Ni(OH)2/GCE possesses a high activity and an excellent stability towards methanol oxidation. NiCo2O4/Ni(OH)2 could also meet the requirements of long-term stability and high activity, which is essential for the anodic materials in DMFCs because of its unique 3D hollow nanoflower-like structure and large SSA.
4. Conclusion
NiCo2O4/Ni(OH)2 nanocomposite was successfully prepared through a simple and fast microwave-assisted synthesis and homogeneous precipitation. The characteristics of NiCo2O4/Ni(OH)2 toward methanol oxidation were investigated. NiCo2O4/Ni(OH)2/GCE exhibited a high electrocatalytic activity and an excellent long-term stability. The performance of NiCo2O4/Ni(OH)2/GCE is attributable to the remarkable electrocatalytic activity, which results from the hierarchical morphology of NiCo2O4 nanoflowers and the high activities of Ni(OH)2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by National Natural Science Foundation of China (No. 21301042, 51361009, and 51461014).References
- L. Carrette, K. A. Friedrich and U. Stimming, ChemPhysChem, 2000, 1, 162–193 CrossRef CAS PubMed.
- H.-L. Liu, F. Nosheen and X. Wang, Chem. Soc. Rev., 2015, 44, 3056–3078 RSC.
- S. P. Jiang, Z. Liu and Z. Q. Tian, Adv. Mater., 2006, 18, 1068–1072 CrossRef CAS.
- S.-H. Ye, J.-X. Feng, A.-L. Wang, H. Xu and G.-R. Li, J. Mater. Chem. A, 2015, 3, 23201–23206 CAS.
- L. Nan, Z. Fan, W. Yue, Q. Dong, L. Zhu, L. Yang and L. Fan, J. Mater. Chem. A, 2016, 4, 8898–8904 CAS.
- N. S. Porter, H. Wu, Z. Quan and J. Fang, Acc. Chem. Res., 2013, 46, 1867–1877 CrossRef CAS PubMed.
- L. Yang, Y. Tang, D. Yan, T. Liu, C. Liu and S. Luo, ACS Appl. Mater. Interfaces, 2016, 8, 169–176 CAS.
- R. Kiyani, S. Rowshanzamir and M. J. Parnian, Energy, 2016, 113, 1162–1173 CrossRef CAS.
- S. Das, K. Dutta and P. P. Kundu, J. Mater. Chem. A, 2015, 3, 11349–11357 CAS.
- W. Zhong and J. Jiang, J. Phys. Chem. C, 2015, 119, 19287–19296 CAS.
- L. Pastor-Pérez and A. Sepúlveda-Escribano, Fuel, 2017, 194, 222–228 CrossRef.
- Y. Liu, Y. Huang, Y. Xie, Z. Yang, H. Huang and Q. Zhou, Chem. Eng. J., 2012, 197, 80–87 CrossRef CAS.
- Z. Xu, Y. Zhu, L. Bai, Q. Lang, W. Hu, C. Gao, S. Zhong and S. Bai, Inorg. Chem. Front., 2017, 4, 1704–1713 RSC.
- Y. Hu, H. Zhang, P. Wu, H. Zhang, B. Zhou and C. Cai, Phys. Chem. Chem. Phys., 2011, 13, 4083–4094 RSC.
- C. Comninellis and C. Pulgarin, J. Appl. Electrochem., 1993, 23, 108–112 CrossRef CAS.
- L. Liao, H. Mai, Q. Yuan, H. Lu, J. Li, C. Liu, C. Yan, Z. Shen and T. Yu, J. Phys. Chem. C, 2008, 112, 9061–9065 CAS.
- G. R. M. Tomboc, A. H. Tamboli and H. Kim, Energy, 2017, 121, 238–245 CrossRef CAS.
- S. Chen, M. Chi, Z. Yang, M. Gao, C. Wang and X. Lu, Inorg. Chem. Front., 2017, 4, 1621–1627 RSC.
- C. Ding, Y. Ma, X. Lai, Q. Yang, P. Xue, F. Hu and W. Geng, ACS Appl. Mater. Interfaces, 2017, 9, 18170–18177 CAS.
- S. Wang, D. Huang, S. Xu, W. Jiang, T. Wang, J. Hu, N. Hu, Y. Su, Y. Zhang and Z. Yang, Phys. Chem. Chem. Phys., 2017, 19, 19043–19049 RSC.
- J. Guo, Q. Liu, C. Wang and M. R. Zachariah, Adv. Funct. Mater., 2012, 22, 803–811 CrossRef CAS.
- Y. Li, P. Hasin and Y. Wu, Adv. Mater., 2010, 22, 1926–1929 CrossRef CAS PubMed.
- B. Lu, D. Cao, P. Wang, G. Wang and Y. Gao, Int. J. Hydrogen Energy, 2011, 36, 72–78 CrossRef CAS.
- Y. Lei, J. Li, Y. Wang, L. Gu, Y. Chang, H. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 1773–1780 CAS.
- P. Boldrin, A. K. Hebb, A. A. Chaudhry, L. Otley, B. Thiebaut, P. Bishop and J. A. Darr, Ind. Eng. Chem. Res., 2007, 46, 4830–4838 CrossRef CAS.
- R. Ding, L. Qi, M. Jia and H. Wang, Catal. Sci. Technol., 2013, 3, 3207–3215 CAS.
- M.-C. Liu, L.-B. Kong, C. Lu, X.-M. Li, Y.-C. Luo, L. Kang, X. Li and F. C. Walsh, J. Electrochem. Soc., 2012, 159, A1262–A1266 CrossRef CAS.
- W.-W. Liu, C. Lu, K. Liang and B. K. Tay, J. Mater. Chem. A, 2014, 2, 5100–5107 CAS.
- K. A. Malinger, K. Laubernds, Y.-C. Son and S. L. Suib, Chem. Mater., 2004, 16, 4296–4303 CrossRef CAS.
- L. Gu, L. Qian, Y. Lei, Y. Wang, J. Li, H. Yuan and D. Xiao, J. Power Sources, 2014, 261, 317–323 CrossRef CAS.
- H. J. Kitchen, S. R. Vallance, J. L. Kennedy, N. Tapia-Ruiz, L. Carassiti, A. Harrison, A. G. Whittaker, T. D. Drysdale, S. W. Kingman and D. H. Gregory, Chem. Rev., 2014, 114, 1170–1206 CrossRef CAS PubMed.
- A. Sivanantham, P. Ganesan and S. Shanmugam, Adv. Funct. Mater., 2016, 26, 4661–4672 CrossRef CAS.
- B. Cui, H. Lin, Y.-Z. Liu, J.-B. Li, P. Sun, X.-C. Zhao and C.-J. Liu, J. Phys. Chem. C, 2009, 113, 14083–14087 CAS.
- S. Sahoo, S. Ratha and C. S. Rout, AJEAS, 2015, 8, 371–379 Search PubMed.
- Z.-Q. Liu, Q.-Z. Xu, J.-Y. Wang, N. Li, S.-H. Guo, Y.-Z. Su, H.-J. Wang, J.-H. Zhang and S. Chen, Int. J. Hydrogen Energy, 2013, 38, 6657–6662 CrossRef CAS.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963–969 CrossRef CAS PubMed.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. D. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- S. Gheorghiu and M.-O. Coppens, AIChE J., 2004, 50, 812–820 CrossRef CAS.
- B. Zhao and M. M. Collinson, Chem. Mater., 2010, 22, 4312–4319 CrossRef CAS.
- X. Cui, P. Xiao, J. Wang, M. Zhou, W. Guo, Y. Yang, Y. He, Z. Wang, Y. Yang, Y. Zhang and Z. Lin, Angew. Chem., 2017, 129, 4559–4564 CrossRef.
- S. Thiel, G. Hammerl, A. Schmehl, C. Schneider and J. Mannhart, Science, 2006, 313, 1942–1945 CrossRef CAS PubMed.
- G. Herranz, M. Basletić, M. Bibes, C. Carrétéro, E. Tafra, E. Jacquet, K. Bouzehouane, C. Deranlot, A. Hamzić, J. M. Broto, A. Barthélémy and A. Fert, Phys. Rev. Lett., 2007, 98, 216803 CrossRef CAS PubMed.
- Q. Ke, C. Guan, X. Zhang, M. Zheng, Y. W. Zhang, Y. Cai, H. Zhang and J. Wang, Adv. Mater., 2017, 29, 1604164 CrossRef PubMed.
- B. Dong, M. Li, S. Chen, D. Ding, W. Wei, G. Gao and S. Ding, ACS Appl. Mater. Interfaces, 2017, 9, 17890–17896 CAS.
- X. Han, X. Xie, C. Xu, D. Zhou and Y. Ma, Opt. Mater., 2003, 23, 465–470 CrossRef CAS.
- X. Liu and L. Yu, J. Power Sources, 2004, 128, 326–330 CrossRef CAS.
- D. P. Dubal, G. S. Gund, C. D. Lokhande and R. Holze, ACS Appl. Mater. Interfaces, 2013, 5, 2446–2454 CAS.
- G. Xiao-yan and D. Jian-cheng, Mater. Lett., 2007, 61, 621–625 CrossRef.
- X. Cui, P. Xiao, J. Wang, M. Zhou, W. Guo, Y. Yang, Y. He, Z. Wang, Y. Yang, Y. Zhang and Z. Lin, Angew. Chem., Int. Ed., 2017, 56, 4488–4493 CrossRef CAS PubMed.
- C. Li, J. Wang, S. Feng, Z. Yang and S. Ding, J. Mater. Chem. A, 2013, 1, 8045 CAS.
- J. Marco, J. Gancedo, M. Gracia, J. Gautier, E. Rios and F. Berry, J. Solid State Chem., 2000, 153, 74–81 CrossRef CAS.
- S. Huang, G.-N. Zhu, C. Zhang, W. W. Tjiu, Y.-Y. Xia and T. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 2242–2249 CAS.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. L. Wang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- P. Hermet, L. Gourrier, J.-L. Bantignies, D. Ravot, T. Michel, S. Deabate, P. Boulet and F. Henn, Phys. Rev. B: Condens. Matter, 2011, 84, 235211 CrossRef.
- Y. L. Lo and B. J. Hwang, Langmuir, 1998, 14, 944–950 CrossRef CAS.
- H. Guo, L. Liu, T. Li, W. Chen, J. Liu, Y. Guo and Y. Guo, Nanoscale, 2014, 6, 5491–5497 RSC.
- R. Ding, L. Qi, M. Jia and H. Wang, Nanoscale, 2014, 6, 1369–1376 RSC.
- G. Hu, C. Tang, C. Li, H. Li, Y. Wang and H. Gong, J. Electrochem. Soc., 2011, 158, A695–A699 CrossRef CAS.
- E. Umeshbabu and G. Ranga Rao, Electrochim. Acta, 2016, 213, 717–729 CrossRef CAS.
- M. Yu, J. Chen, J. Liu, S. Li, Y. Ma, J. Zhang and J. An, Electrochim. Acta, 2015, 151, 99–108 CrossRef CAS.
- M. Asgari, M. G. Maragheh, R. Davarkhah and E. Lohrasbi, J. Electrochem. Soc., 2011, 158, K225–K229 CrossRef CAS.
- N. Spinner and W. E. Mustain, Electrochim. Acta, 2011, 56, 5656–5666 CrossRef CAS.
- W. Wang, Q. Chu, Y. Zhang, W. Zhu, X. Wang and X. Liu, New J. Chem., 2015, 39, 6491–6497 RSC.
- A. K. Das, R. K. Layek, N. H. Kim, D. Jung and J. H. Lee, Nanoscale, 2014, 6, 10657–10665 RSC.
- R. Ding, L. Qi, M. Jia and H. Wang, J. Power Sources, 2014, 251, 287–295 CrossRef CAS.
- M. Asgari, M. G. Maragheh, R. Davarkhah, E. Lohrasbi and A. N. Golikand, Electrochim. Acta, 2012, 59, 284–289 CrossRef CAS.
- A. Mahajan, S. Banik, P. S. Roy, S. R. Chowdhury and S. K. Bhattacharya, Int. J. Hydrogen Energy, 2017, 42, 21263–21278 CrossRef CAS.
- Y. Song, X. Zhang, S. Yang, X. Wei and Z. Sun, Fuel, 2016, 181, 269–276 CrossRef CAS.
- X. Cui, W. Guo, M. Zhou, Y. Yang, Y. Li, P. Xiao, Y. Zhang and X. Zhang, ACS Appl. Mater. Interfaces, 2014, 7, 493–503 Search PubMed.
- L. Gu, L. Qian, Y. Lei, Y. Wang, J. Li, H. Yuan and D. Xiao, J. Power Sources, 2014, 261, 317–323 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qi00583k |
| This journal is © the Partner Organisations 2018 |