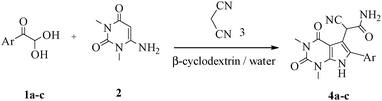
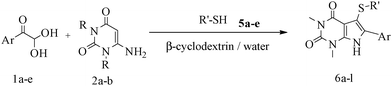
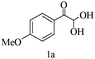
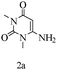
A green route for the synthesis of pyrrolo[2,3-d]pyrimidine derivatives catalyzed by β-cyclodextrin†
Vijay B.
Yadav
,
Pragati
Rai
,
Hozeyfa
Sagir
,
Akhilesh
Kumar
and
I. R.
Siddiqui
 *
*
Laboratory of Green Synthesis, Department of Chemistry, University of Allahabad, Allahabad, 211002, India. E-mail: dr.irsiddiqui@gmail.com; Tel: +91-9335153359
First published on 22nd November 2017
Abstract
Herein, we present the synthesis of pyrrolo[2,3-d]pyrimidine derivatives, which are important heterocyclic scaffolds in the field of synthetic and pharmaceutical research, via biomimetic catalysis. This strategy involves the use of β-cyclodextrin as a reusable promoter and water as an eco-friendly reaction medium. The merits of this protocol are high atom economy, mild reaction conditions, good yields of desired products in short reaction times, and reusable reaction medium.
Introduction
Supramolecular catalysis is an area in chemical science involving intermolecular interactions between interacting substrates, which can be molecules, ions or radicals. The most accessible β-cyclodextrin is cyclic oligosaccharides, which possess the ability to bind reactants selectively and promote chemical reactions with great efficiency. They are cyclic heptamers that have become extensively exploited members in the cyclodextrin group,1 and their exclusive property of a hydrophilic peripheral and a hydrophobic cavity make them remarkable promoters in organic synthesis. They catalyze chemical reactions by supramolecular catalysis involving reversible construction of host–guest relations via non-covalent bonding such as that in enzymes.2 This type of interaction between β-CD units and reactants intensifies the local concentration of substrate and keeps the substrate near the catalytic active center; hence, this accelerates the rate of reaction and provides excellent substrate selectivity.3 Biomimetic reactions can be efficiently performed in aqueous media without the production of any hazardous waste products and thus accurately mimic biocatalytic environments. Consequently, cyclodextrins are acknowledged as enzyme models, and chemical reactions under supramolecular catalysis are preferable to chemical catalysis. These properties encouraged us to exploit this catalyst in the development of pyrrolo[2,3-d]pyrimidine scaffolds starting from amino uracil, malononitrile, and substituted aldehydes.Pyrrolo[2,3-d]pyrimidine is a basic structural feature widely distributed in several natural products as well as in synthetic drugs.4 Pyrrolo[2,3-d]-pyrimidine is structurally similar to purines and pyrimidines, which shows varied biological activities such as antitumor,5 antiviral,6 antifolate,7 antagonist to receptors,8 analgesic,9 antimicrobial,10 antifungal,11 anticancer,12 antibiotic,13 and antiproliferative.14 In addition, its derivatives are powerful inhibitors of protein kinases, such as the enzyme Janus kinase 3 (JAK 3), and therefore, beneficial for the treatment of several immunological syndromes.15 Various clinically permitted medicines have pyrrolo[2,3-d]-pyrimidine nuclei, for example, pemetrexed, used in chemotherapy for the treatment of pleural mesothelioma and lung cancer,16 and to facitinib, utilized in the treatment of rheumatoid arthritis (RA), psoriasis, and inflammatory bowel disease.17 Pyrrolo[2,3-d]pyrimidine and its derivatives also display strong UV-blue fluorescence and are used as fluorescent functional resources.18 Thus, considering their wide applications, various methods have been reported for the synthesis of this targeted moiety. However, most of these protocols suffer from several limitations such as the use of expensive, toxic, and non-recyclable catalysts, long reaction times, tedious work-ups, and formation of undesirable side products.19 Therefore, we decided to develop a more convenient and efficient synthetic procedure for the synthesis of the abovementioned moiety.
Results and discussion
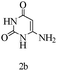
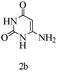
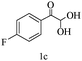
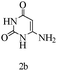
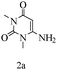
Herein, we disclosed a biomimetic path for the first time for the synthesis of pyrrolo[2,3-d]-pyrimidine by employing 6-aminouracil, malononitrile, and arylglyoxal monohydrate in aqueous β-cyclodextrin (Scheme 1).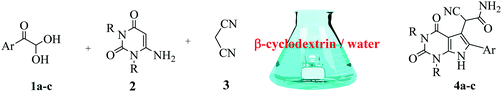 | ||
| Scheme 1 Synthesis of pyrrolo[2,3-d]-pyrimidine in the presence of β-cyclodextrin in an aqueous medium. | ||
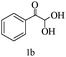
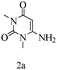
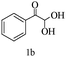
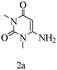
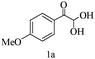
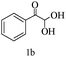
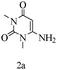
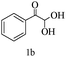
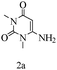
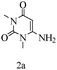
Preliminary investigations on the title reaction were performed using arylglyoxal monohydrate (1a), malononitrile (2), and 6-amino-1,3-dimethyluracil (3) as model substrates to optimize the reaction conditions (Scheme 2).
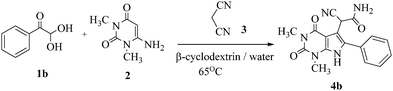 | ||
| Scheme 2 The model reaction to optimize the reaction conditions for the synthesis of pyrrolo[2,3-d]-pyrimidine. | ||
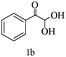
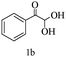
For optimization, the chemical reaction was carried out under several conditions. Initially, we optimized the catalyst for this transformation. When the reaction was carried out in the absence of β-cyclodextrin, a very low yield of the product was obtained in a longer reaction time. Further, in the presence of an ionic liquid, no significant increase in the yield was observed, and the time taken for the completion of the reaction was 5 hours (Table 1, entry 2). Cerric ammonium nitrate and bismuth triflate (Table 1, entries 3 and 4) provided a better yield of products than the ionic liquid. Malic acid provided higher yields as compared to the abovementioned catalysts (Table 1, entry 5). To our delight, a noticeable improvement in the yield of the product was observed when the reaction occurred in the presence of β-cyclodextrin (Table 1, entry 8).
| Entry | Catalyst (10 mol%) | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: arylglyoxal monohydrate (1.0 mmol), malononitrile (1.0 mmol) and 6-amino-1,3-dimethyluracil (1.0 mmol), in water. b Isolated yield of product. | |||
| 1 | No catalyst | 5.0 | 21 |
| 2 | [Bmim]Br | 5.0 | 35 |
| 3 | Cerric ammonium nitrate | 5.0 | 41 |
| 4 | Bismuth(III) triflate | 5.0 | 47 |
| 5 | Malic acid | 5.0 | 49 |
| 6 | α-Cyclodextrin | 4.0 | 51 |
| 7 | γ-Cyclodextrin | 5.0 | — |
| 8 | β-Cyclodextrin | 1.5 | 89 |
After optimization of the catalyst, we optimized the loading amount of the catalyst. The best result was obtained for 10 mol% of catalyst that provided excellent yields of the corresponding products within 1.5 hours under aqueous conditions. However, an increase in the amount of catalyst did not result in any improvement in the yield of the product (Table 2, entry 3). The time taken for completion of the reaction was also the same. All the results are presented in Table 2.
Next, we optimized the temperature and found that temperature played an important role. When the reaction was carried out at room temperature, only a trace of product was obtained after a long reaction time (Table 3, entry 1). Subsequently, the temperature was increased from 40 °C to 75 °C. It was found that the yield of the product increased upon increasing the temperature, and 65 °C (Table 3, entry 3) was found to be optimum for this conversion.
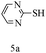
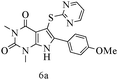
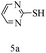
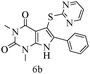
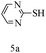
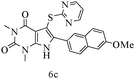
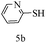
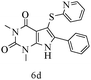
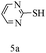
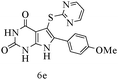
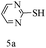
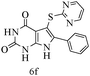
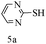
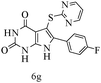
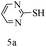
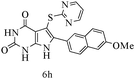
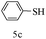
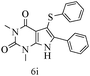
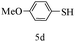
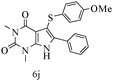
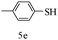
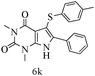
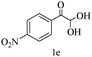
After optimizing the reaction conditions, a variety of pyrrolo[2,3-d]pyridinedione derivatives were constructed, and the results are listed in Table 4. It was observed that various types of arylglyoxals having substitution on the phenyl ring undergo a smooth transformation via this proposed protocol. After the successful demonstration of this strategy, the scope of this methodology was extended with several thiols, and pyrrolo[2,3-d]pyrimidinedione derivatives were formed in good yields (Table 4, entry 4). Water was used as the reaction medium for all the entries at 65 °C. In all the cases, the yield of the product was good. The structures of all the corresponding products were confirmed via1H NMR and 13C NMR spectroscopies and mass spectrometry.
| Entry | 1a–c | 4a–c | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: arylglyoxal monohydrate (1.0 mmol), malononitrile (1.0 mmol), and 6-amino-1,3 dimethyl uracil (1.0 mmol), in water with beta cyclodextrin (10 mol%) at 65 °C. b Isolated yield of product. c Reaction conditions: arylglyoxal monohydrate (1.0 mmol), thiols (1.0 mmol), and 6-amino-1,3 dimethyl uracil (1.0 mmol), in water with β-cyclodextrin (10 mol%) at 65 °C. d Isolated yield of product. | |||
| 1 |
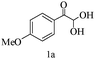
|
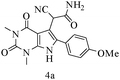
|
85 |
| 2 |
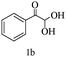
|
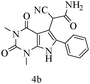
|
89 |
| 3 |
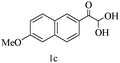
|
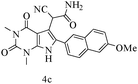
|
88 |
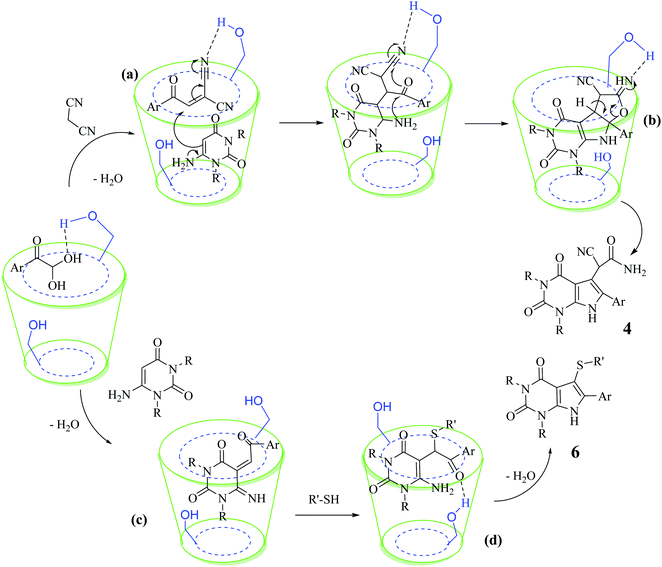
Based on the abovementioned results and in accordance with the literature survey, a plausible reaction mechanism is proposed that illustrates the role of β-CD in the synthesis of pyrrolo[2,3-d]-pyrimidine. According to the literature and our experimental results, the mechanism proceeds via the activation of arylglyoxal by the OH group of β-cyclodextrin due to hydrogen bonding. In the first step, malononitrile reacts with arylglyoxal to provide the Knoevenagel product (a) that undergoes a Michael addition reaction with 6-aminouracil to form the adduct (b); the adduct (b) then undergoes ring opening to form the compound (6). The same pathway is followed to form adduct (c). Various thiols undergo nucleophilic addition to form compound (d), which further undergoes a cyclisation reaction to the form the desired products (5) (Scheme 3).
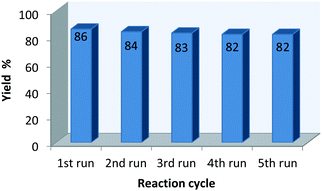
Recovery of the catalyst system
After the completion of the reaction, the corresponding solid product was isolated by simple filtration, and the filtrate was utilized for next cycle. The recycled β-CD was utilized in the reaction with the same substrates, and the results are shown in Fig. 1. The reusability of the catalyst was studied for five reaction cycles (using the fresh catalyst) for the synthesis of compounds, and only a marginal decrease in the yield of the desired product was observed.Conclusions
In summary, we have reported the first example of β-cyclodextrin-promoted synthesis of pyrrolo[2,3-d]-pyrimidine in aqueous media. This method provides a green route for the synthesis of targeted scaffolds and also a wide substrate scope for several substituted aldehydes to provide good yields of the corresponding products. Furthermore, the catalyst can be easily recovered by simple filtration and reused several times without any substantial loss in activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors V. B. Yadav, A. Kumar and H. Sagir thank CSIR and UGC, New Delhi, for offering them the Junior Research Fellowship (JRF). P. Rai thanks CSIR for providing the Senior Research Fellowship (SRF). Author also thank SAIF, Chandigarh, for providing spectral data of the synthesised compounds.Notes and references
- (a) J. Szejtli, Chem. Rev., 1998, 98, 1743 CrossRef CAS PubMed; (b) S. V. Bhosale, Mini-Rev. Org. Chem., 2007, 4, 143 CrossRef.
- (a) R. Breslowong and S. D. Dong, Chem. Rev., 1998, 98, 1997 CrossRef; (b) J. M. Desper and J. Breslow, J. Am. Chem. Soc., 1994, 116, 12081 CrossRef CAS.
- (a) J. Bjerre, T. H. Fenger, L. G. Marinescu and M. Bols, Eur. J. Org. Chem., 2007, 704 CrossRef CAS; (b) L. G. Marinescu, E. G. Doyagueez, M. Petrillo, A. Fernandez-Mayoralas and M. Bols, Eur. J. Org. Chem., 2010, 157 CrossRef CAS; (c) M. Zhao, H. L. Wang, L. Zhang, C. Y. Zhao, L. N. Ji and Z. W. Mao, Chem. Commun., 2011, 47, 7344 RSC; (d) S. P. Tang, S. Chen, G. F. Wu, H. Y. Chen, Z. W. Mao and L. N. Ji, Inorg. Chem. Commun., 2011, 14, 184 CrossRef CAS.
- (a) P. K. Gupta, S. Daunert, M. R. Nassiri, L. L. Wotring, J. C. Drach and L. B. Townsend, J. Med. Chem., 1989, 32, 402 CrossRef CAS PubMed; (b) Q. Dang and J. E. Gomez-Galeno, J. Org. Chem., 2002, 67, 8703 CrossRef CAS PubMed; (c) L. M. De Coen, T. S. A. Heugebaert, D. García and C. V. Stevens, Chem. Rev., 2016, 116, 80 CrossRef CAS PubMed.
- (a) T. Miwa, T. Hitaka, H. Akimoto and H. Nomura, J. Med. Chem., 1991, 34, 555 CrossRef CAS PubMed; (b) X. Y. Jiao, D. J. Kopecky, J. S. Liu, J. Q. Liu, J. C. Jaen, M. G. Cardozo, R. Sharma, N. Walker, H. Wesche, S. Li, E. Farrelly, S. H. Xiao, Z. Wang and F. Kayser, Bioorg. Med. Chem. Lett., 2012, 22, 6212 CrossRef CAS PubMed.
- J. S. Pudlo, M. R. Nassiri, E. R. Kern, L. L. Wotring, J. C. Drach and L. B. Townsend, J. Med. Chem., 1990, 33, 1984 CrossRef CAS PubMed.
- A. Gangjee, Y. Zeng, J. J. McGuire, F. Mehraein and R. L. Kisliuk, J. Med. Chem., 2004, 47, 6893 CrossRef CAS PubMed.
- S. Hess, C. E. Muller, W. Frobenius, U. Reith, K. N. Klotz and K. Eger, J. Med. Chem., 2000, 43, 4636 CrossRef CAS PubMed.
- S. H. Boyer, B. G. Ugarkar, J. Solbach, J. Kopcho, M. C. Matelich, K. Ollis, J. E. Gomez-Galeno, R. Mendonca, M. Tsuchiya, A. Nagahisa, M. Nakane, J. B. Wiesner and M. D. Erion, J. Med. Chem., 2005, 48, 6430 CrossRef CAS PubMed.
- M. S. Mohamed, R. Kamel and S. Fatahala, Eur. J. Med. Chem., 2010, 45, 2994 CrossRef CAS PubMed.
- M. S. A. El-Gaby, A. M. Gaber, A. A. Atalla and K. A. Abd Al-Wahab, Farmaco, 2002, 57, 613 CrossRef CAS PubMed.
- (a) M. M. Ghorab, F. A. Ragab, H. I. Heiba, H. A. Youssef and M. G. El-Gazzar, Bioorg. Med. Chem. Lett., 2010, 20, 6316 CrossRef CAS PubMed; (b) S. J. Tangeda and A. Garlapati, Eur. J. Med. Chem., 2010, 45, 1453 CrossRef CAS PubMed.
- (a) R. L. Tolman, R. K. Robins and L. B. Townsend, J. Am. Chem. Soc., 1968, 90, 524 CrossRef CAS PubMed; (b) S. H. Krawczyk, M. R. Nassiri, L. S. Kucera, E. R. Kern, R. G. Ptak, L. L. Wotring, J. C. Drach and L. B. Townsend, J. Med. Chem., 1995, 38, 4106 CrossRef CAS PubMed.
- M. H. Jung, H. Kim, W. K. Choi, M. I. El-Gamal, J. H. Park, K. H. Yoo, T. B. Sim, S. H. Lee, D. Baek, J. M. Hah and J. H. Cho, Bioorg. Med. Chem. Lett., 2009, 19, 6538 CrossRef CAS PubMed.
- T. A. Blumenkopf, M. E. Flanagan and M. J. Munchhof, Int. Patent, WO 2001042246 A2, 2001 Search PubMed.
- C. Manegold, G. Schmid-Bindert and L. R. Pilz, Expert Rev. Anticancer Ther., 2009, 9, 1195 CrossRef CAS PubMed.
- (a) C. A. Zerbini and A. B. Lomonte, Expert Rev. Clin. Immunol., 2012, 8, 319 CrossRef CAS PubMed; (b) M. Galluzzo, S. D’Adamio, S. Servoli, L. Bianchi, S. Chimenti and M. Talamonti, Expert Opin. Pharmacother., 2016, 17, 1421 CrossRef CAS PubMed.
- S. Tumkevicius, J. Dodonova, K. Kazlauskas, V. Masevicius, L. Skardziute and S. Jursenas, Tetrahedron Lett., 2010, 51, 3902 CrossRef CAS.
- J. A. Secrist III and P. S. Liu, J. Org. Chem., 1978, 43, 3937 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nj03577b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |