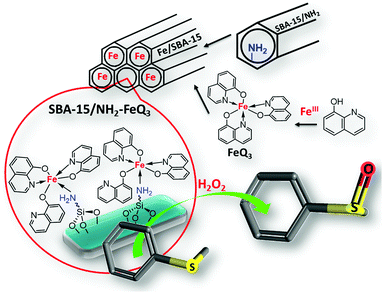
Post-synthetically modified SBA-15 with NH2-coordinately immobilized iron-oxine: SBA-15/NH2-FeQ3 as a Fenton-like hybrid catalyst for the selective oxidation of organic sulfides
Habib
Golchin Hosseini
and
Sadegh
Rostamnia
 *
*
Organic and Nano Group (ONG), Department of Chemistry, University of Maragheh, P.O. Box 55181-83111, Maragheh, Iran. E-mail: rostamnia@maragheh.ac.ir; srostamnia@gmail.com; Tel: +98 4212278001-108
First published on 24th November 2017
Abstract
A new modified SBA-15 meso-material with NH2-coordinately immobilized “iron-oxine” was fabricated by stepwise covalent grafting of -NH2 and then NH2-coordinate immobilization of tris(8-quinolinolato)iron onto the channels of SBA-15. The structural properties and chemical nature of SBA-15/NH2-FeQ3 were characterized by FTIR, scanning electron microscopy (SEM), SEM-EDX/mapping, transmission electron microscopy (TEM), low angle X-ray diffraction (L-XRD), thermal gravimetric analysis (TGA), N2-adsorption–desorption isotherms (BET) and atomic absorption spectroscopy (AAS). A Fenton-like green and eco-friendly procedure for the catalytic oxidation of aromatic organic sulfides to sulfones using H2O2 was investigated for the synthesised Fe/SBA-15 catalyst. This porous catalyst was found to be reusable for ten runs without appreciable change in the activity, or the composition of the catalyst.
1. Introduction
Post-synthetically modified ordered mesoporous silica (OMS) materials, constructed from repeating organic linkers or metal–ligand functional groups in the mesochannels, are attractive because they can be made from readily available and highly tailorable ligand precursors for multifunctional integration.1–3 The topological structure of OMS is fully ordered in nature and leads to regular porosity, and the functional ligand species can also be vastly expanded and thus render versatile utility, including in catalysis, separation, gas storage, supercapacitance, and biomolecule and drug delivery.1,4–10 With this in mind, designing a catalytically active covalently-bonded ligand OMS (OMS/L) targeting a specific reaction should be possible. Due to the strong metal–ligand bonds, OMS/L acts as a scaffold to support transition metal-based catalysts and allows for a large interfacial surface area, where the catalytic active sites of the transition metal can be remarkably exposed.10 Furthermore, conjugation of OMS/L with other functional ligands is easily achieved and elaborately modulated. The produced hybrids have well-defined organic–inorganic hybrid mesostructures, expanding their scope of utilization.8,11Due to the importance of sulfoxides in biology and as intermediates in industry, the oxidation of sulfides to the corresponding sulfoxides has been a focus of considerable research.12,13 In this field, many oxidants such as molecular Oxone, UHP, TBHP, and H2O2 are available to perform this reaction.14–19 The overoxidation of sulfides resulting in sulfone formation is a common problem. In this field, intensive effort has been devoted to the development of oxidation reactions using green reagents and solid catalysts that co-produce only innocuous waste.20,21 For example, oxidation using aqueous H2O2 that produces only water as a co-product is a topic of current interest, and quite a few atom-efficient methods using H2O2 have been reported.
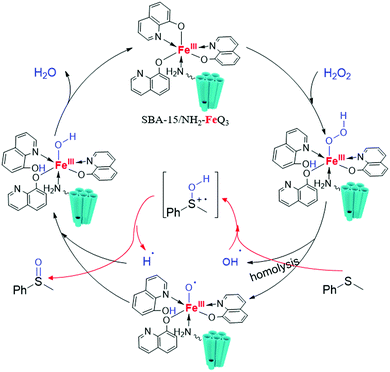
Previously, we developed synthetic methods for the oxidation process and also the synthesis of interesting organic molecules.22–30 The challenge in this field is the development of efficient, green and selective methods.30 Moreover, the synthesis of these molecules has usually been carried out in hazardous and polar solvents such as CCl4, C6H6, and DMSO, leading to complex isolation and recovery procedures. Recently, we prepared a series of OMS/L hybrids based on SBA-15 materials by a post-synthetic modification method, which displayed comparable activity and selectivity to homogeneous systems in a wide range of organic reactions.30 Encouraged by these successful efforts and aiming to develop a stable and selective green catalyst, herein we report the synthesis of SBA-15/NH2-FeQ3via a stepwise post-synthetic modification method and then we aimed to examine the catalytic activity of SBA-15/NH2-FeQ3 in the oxidation of sulfides to sulfoxides. Herein, FeCl3 was chelated with tris(8-quinolinolato), and the formed tris(8-quinolinolato)iron complexes (FeQ3 or Fe-oxine) were deposited/coordinated onto SBA-15/NH2 to yield SBA-15/NH2-FeQ3 as a new, tunable, and highly ordered mesoporous Fe/SBA-15 silica. The use of our proposed catalytic system for the selective oxidation of sulfides has surpassed the previous methods and the drawbacks of the previously reported methods have been resolved. This system could be used under waste-free conditions with high selectivity towards sulfoxides (Fig. 1).
2. Experimental
2.1 Materials and apparatus
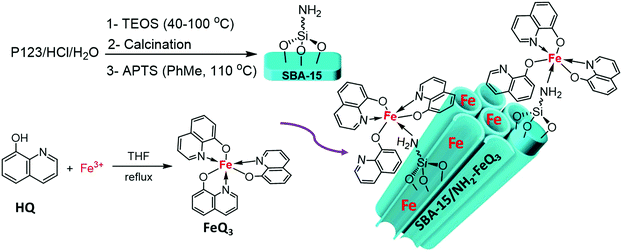
Triblock copolymer Pluronic P123, tetraethoxysilane (TEOS), (3-aminopropyl)triethoxysilane [(EtO)3SiPrNH2, APTS], 8-hydroxyquinoline (oxyquinoline), FeCl3·6H2O and other reagents were obtained from Aldrich, Merck (Germany) and Fluka (Switzerland) and used as received. Melting points were measured on Electrothermal 9100 apparatus. The IR spectra were determined using a FT-IR Bruker-Vector 22. Homogeneous stirring was achieved by ultrasonication (Ultrasonic Homogenizer-model APU500 Advanced Equipment Engineering Company-Adeeco, Iran). Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) were performed using a VEGA3 TESCAN. Powder X-ray diffraction spectra were recorded on a Philips PW1800. Transmission electron microscopy (TEM) was performed on a JOEL JEM 3010 instrument.2.2. Synthesis of tris(8-quinolinolato)iron (FeQ3 or iron-oxine)
The tris(8-quinolinolato)iron (FeQ3) was synthesized by taking 8-hydroxyquinoline (436 mg, 3 mmol) and 1 mmol (270 mg) FeCl3·6H2O in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and stirring at 65 °C in 10 mL of anhydrous THF for 4 h under a nitrogen atmosphere in a three-necked Schlenk flask. After cooling the reaction mixture to room temperature, a precipitate appeared, and this solid was filtered and washed 3 times with cold THF.
1 molar ratio and stirring at 65 °C in 10 mL of anhydrous THF for 4 h under a nitrogen atmosphere in a three-necked Schlenk flask. After cooling the reaction mixture to room temperature, a precipitate appeared, and this solid was filtered and washed 3 times with cold THF.
2.3. Synthesis of NH2-functionalized SBA-15 (SBA-15/NH2)
The mesoporous SBA-15 material was synthesized according to our previously reported procedure.23,31,32 NH2-Functionalized SBA-15 was synthesised by adding 2000 μL of APTES to a sonicated suspension (2 × 5 min) of calcinated SBA-15 (1 g) in toluene at 110 °C for 24 h. After the reaction stopped, the temperature was decreased to room temperature and the solid was filtered and washed with ethanol. Finally, SBA-15/NH2 was dried under vacuum at 60 °C.2.4. SBA-15/NH2-Coordinately immobilized iron-oxine (SBA-15/NH2-FeQ3)
The obtained SBA-15/NH2 was then incorporated with tris(8-quinolinolato)iron. 1 mg of the obtained FeQ3 was dissolved in 50 mL of CHCl3, followed by addition of 1 g of SBA-15/NH2 under vigorous stirring, and then the suspension was refluxed for 24 h. Finally, the solid was collected by filtration and Soxhlet extraction with EtOH for 72 h, and then after two rounds of sonication for around 2 min in chloroform, it was dried at 60 °C under vacuum. According to atomic absorption analysis (AAS) of the composite, the amount of loaded Fe3+ was 0.6 mmol per gram of composite.2.5. General procedure for the oxidation of sulfides
In a round-bottomed flask (25 mL) equipped with a magnetic stirrer, sulfide (0.5 mmol) was added into the reaction flask in the presence of 1.5 mmol H2O2 (30% w/w) and SBA-15/NH2-FeQ3 (5 mol% based on FeIII) in 1 mL of water and stirred at 25 °C for an appropriate time (Table 1). After completion of the reaction, the product was extracted from the reaction mixture with chloroform. Then, it was evaporated and analyzed by gas chromatography (GC).| Entry | Ar | R | Time (h) | Yieldsb (%) |
|---|---|---|---|---|
| a Reaction conditions: reaction scale (0.5 mmol). b Yields based on GC analysis (sulfoxide/sulfone). | ||||
| 1 | Ph | PhCH2 | 3 | 90/4 |
| 2 | Ph | Ph | 9 | 44/0 |
| 3 | 4-Br-Ph | PhCH2 | 1 | 75/12 |
| 4 | Ph- | CH3 | 3 | 87/10 |
| 5 | Ph | 2-Py | 4 | 65/27 |
| 6 | Ph | 3-MeO-Ph | 4 | 67/9 |
| 7 | 3-MeO-Ph | 3-MeO-Ph | 4 | 73/9 |
| 8 | PhCH2- | PhCH2- | 2 | 88/6 |
| 9 | 4-Br-Ph | 4-MeO-Ph | 3 | 75/7 |
3. Results and discussion
The SBA-15/NH2-FeQ3 was synthesized by taking 8-hydroxyquinoline and ferric chloride in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in THF and mixing this with amine-grafted SBA-15. Here, the FeQ3 was dissolved in chloroform, followed by the addition of SBA-15/NH2 under stirring and then refluxing the suspension. Finally, the SBA-15/NH2-FeQ3 was collected and dried for further use (Scheme 1).
1 molar ratio in THF and mixing this with amine-grafted SBA-15. Here, the FeQ3 was dissolved in chloroform, followed by the addition of SBA-15/NH2 under stirring and then refluxing the suspension. Finally, the SBA-15/NH2-FeQ3 was collected and dried for further use (Scheme 1).
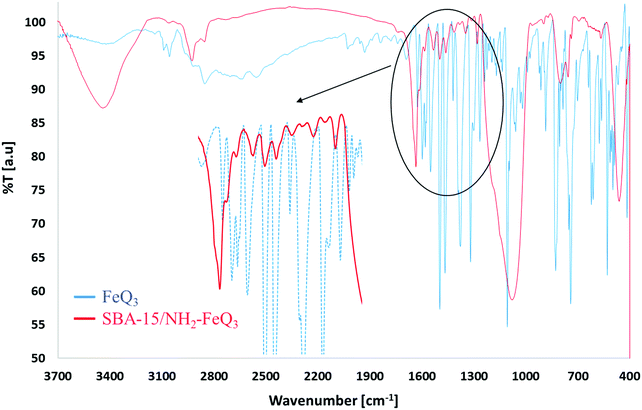
The physicochemical properties of the SBA-15/NH2-FeQ3 were characterized by FTIR, scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (SEM-EDS), SEM mapping, transmission electron microscopy (TEM), low angle X-ray diffraction (XRD), thermal gravimetric analysis (TGA), N2-adsorption–desorption isotherm analysis (BET) and atomic absorption spectroscopy (AAS). FTIR spectroscopy was performed for every step of the catalyst synthesis and the changes in the vibration modes were carefully monitored (Fig. 2). For FeQ3 and SBA-15/NH2-FeQ3, the band in the aromatic region at 1650–1450 cm−1 can be correlated to the splitting of the characteristic absorption of FeQ3 at 1643–1455 cm−1, which shifts to a lower wavenumber. In SBA-15/NH2-FeQ3, the bands at ∼1000 and ∼1100 cm−1 belong to the vibrations of (Si–O–Si) bonds, and the SiO–H groups are shown by the very broad IR absorption band in the 3420–3600 cm−1 region. The peaks at 2800–2940 cm−1 can be attributed to the presence of C–H of the alkyl chain of the propyl group and C–H of the oxine ligand in the samples. The peak at 1465 cm−1 can probably be attributed to the presence of quaternary amines. The SiO–H band appears at around 3300 cm−1.
SEM micrographs of the SBA-15/NH2-FeQ3 nanomaterial are shown in Fig. 3a and b, and these images were used to examine the morphology and topography of the prepared material. The shape of the magnetic nanomaterial shows that the particles were prepared in a nanospherical-shaped morphology in a stockpiled topology with an average size of 60–80 nm. Then, the image profile and plot profile of a selected area of the SEM image were measured (Fig. 3c and d). The line profile of the nanomaterial surface is given in Fig. 3c. It shows that the particle width was around 2 μm. From the similar profile analysis, other parameters such as the pitch and bit height of the surface are measured (Fig. 3d).
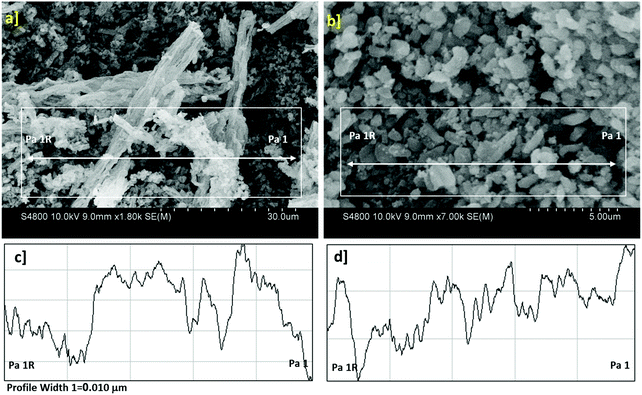 | ||
| Fig. 3 SBA-15/NH2-FeQ3: (a) SEM image and (b) Bin-SEM image, (c) image profile of the selected area highlighted and (d) plot profile of the selected area highlighted. | ||
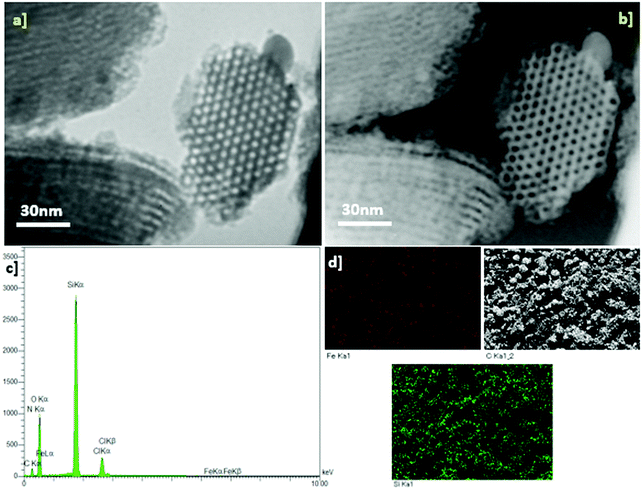
Fig. 4 shows transmission electron microscopy (TEM) images of the as-synthesized SBA-15/NH2-FeQ3 sample. Based on this image, the average size of the channels is mainly around 7 nm. In addition, the TEM images confirmed the presence of a 2D hexagonal network, and no aggregated Fe particle species were observed in the pore channels. This result, together with the elemental mapping images and EDX analysis, indicates homogeneous Fe in a complex format on the SBA-15. EDX analysis clearly indicates the presence of Fe, C, O, N and Si on the SBA-15/NH2-FeQ3 (Fig. 4c).
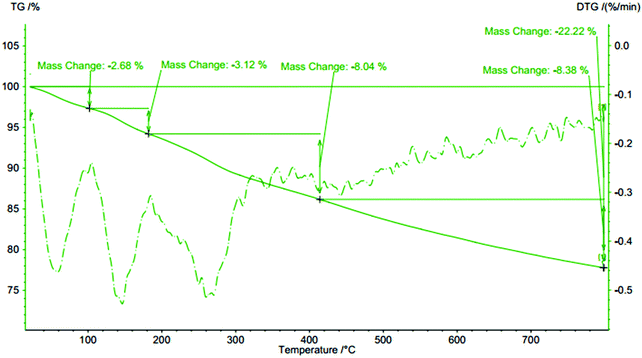
To examine the thermal stability of the SBA-15/NH2-FeQ3, thermal gravimetric analysis (TGA) was carried out between 25 °C and 800 °C in a static atmosphere of nitrogen (Fig. 5). TGA of SBA-15/NH2-FeQ3 shows a weight loss due to the desorption of water below 150 °C, which is due to the loss of inner water. This is finally followed by a set of weight losses centered around 350 °C, corresponding to the elimination of the surface bound organic propyl amine and corresponding coordinated 8-quinolinolato groups, which indicates that this immobilized hybrid catalyst is thermally stable up to 380 °C.
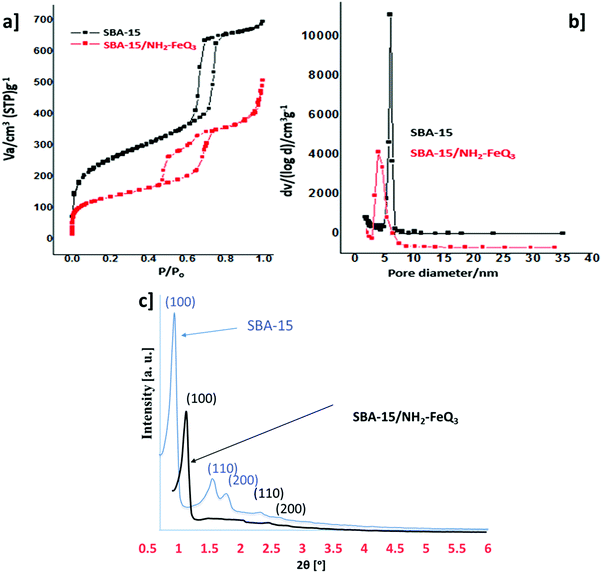
The nitrogen adsorption–desorption isotherms (BET) of SBA-15/NH2-FeQ3 and pure SBA-15 are compared in Fig. 6a. These isotherms reveal that stepwise post-synthetic modification does not perturb the isotherm type of the resulting SBA-15, which remains Type IV. However, the hysteresis loop progressively shifts to lower relative pressures with grafting of propyl amine coordinated FeQ3, indicative of decreasing pore size. The steep step and narrow hysteresis loops in the adsorption/desorption branches evidence retention of the parent SBA-15 cylindrical, parallel pore channels. The BET surface area of SBA-15 was calculated to be ca. 670 m2 g−1. Even after grafting of the FeQ3 complex, a high surface area, ca. 368 m2 g−1, was still retained. BJH pore size distribution analysis of the desorption branch (Fig. 6b) shows that the pore diameter of the mother SBA-15 is 7 nm and a pore diameter of 4.1 nm was achieved in SBA-15/NH2-FeQ3via our method.
Low-angle X-ray diffraction reveals that the SBA-15 and SBA-15/NH2-FeQ3 materials have good long-range order with characteristic (100) diffraction peaks consistent with P6mm hexagonal symmetry (Fig. 6c). According to this comparison, diffraction lines related to Fe3O4 or other crystal/particle species were not present in SBA-15/NH2-FeQ3 due to the nanocomposite formation of silica.
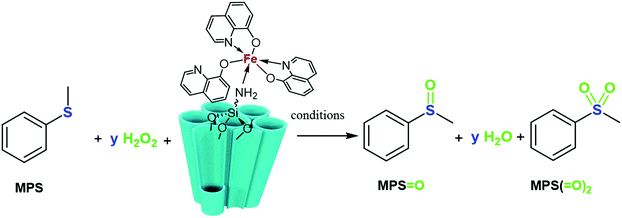
Oxidation of aromatic sulfides is the best alternative to supply industrially interesting intermediates of sulfoxides.16,18 One of the difficulties encountered with this method is the overwhelming overoxidation of the generated sulfoxides (R2S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) to sulfones (R2SO2) in the reaction vessel, without any selectivity, leading to undesired compounds. In this project, the catalytic ability of the SBA-15/NH2-FeQ3 in the oxidation reaction of sulfides to sulfoxides (or sulfones) without using any salts, stabilization or additives was investigated. Oxidation of methyl phenyl sulfide (MPS) as a model benchmark reaction was first examined with SBA-15/NH2-FeQ3 using H2O2 (Scheme 2).
O) to sulfones (R2SO2) in the reaction vessel, without any selectivity, leading to undesired compounds. In this project, the catalytic ability of the SBA-15/NH2-FeQ3 in the oxidation reaction of sulfides to sulfoxides (or sulfones) without using any salts, stabilization or additives was investigated. Oxidation of methyl phenyl sulfide (MPS) as a model benchmark reaction was first examined with SBA-15/NH2-FeQ3 using H2O2 (Scheme 2).
To obtain the optimized conditions, various parameters such as temperature, amount of catalyst, and several solvents were taken into consideration. First, the effect of the amount of SBA-15/NH2-FeQ3 was examined on the model reaction under the following conditions: 25 °C, a reaction time of 2 h, a sulfide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 ratio of 1
H2O2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1 mL of H2O at a reaction scale of 0.5 mmol. Several amounts of catalyst were added to the reaction mixtures and among them 10 mol% of supported FeQ3 was found to be more efficient from the point view of yield in each reaction (Fig. 7).
5 and 1 mL of H2O at a reaction scale of 0.5 mmol. Several amounts of catalyst were added to the reaction mixtures and among them 10 mol% of supported FeQ3 was found to be more efficient from the point view of yield in each reaction (Fig. 7).
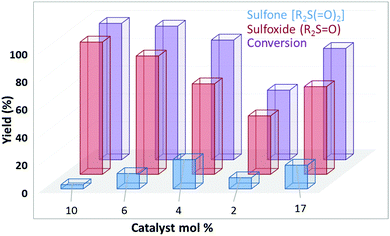 | ||
| Fig. 7 Reaction conditions: 0.5 mmol of MPS, 2.5 mmol of H2O2, in water (1 mL) at 25 °C for 2 h. Violet (conversion), red (sulfoxide) and blue (sulfone). Conversion calculated based on MPS. | ||
The amount of H2O2 oxidant was also optimized for the model oxidation reaction. Based on this study, the best amount of H2O2 for this work was 4 mmol for each mmol of sulfide (room temperature conditions). Fig. 8 represents the results for six different amounts of H2O2 in reactions performed under similar conditions.
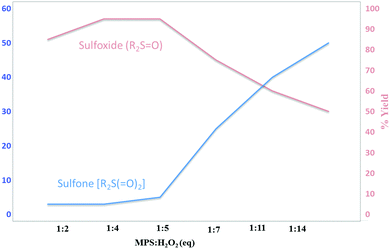 | ||
| Fig. 8 Optimizing the amount of H2O2 in the model reaction. Reaction conditions: 0.5 mmol of MPS in H2O (1 mL) at 25 °C for 2 h. | ||
The effect of temperature on the product yield was also monitored under similar conditions. In this regard, temperatures including 10, 25, 60, and 100 °C of the model reaction were tested. One notable point in this comparison was that the reaction at 25 °C had a lower reaction completion time for the selective synthesis of the sulfoxide with <10% of the sulfone (Fig. 9).
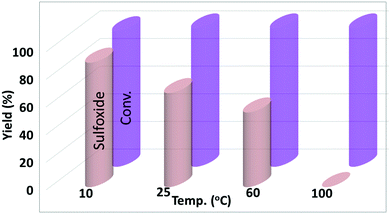 | ||
| Fig. 9 Reaction conditions: 0.5 mmol of MPS, 2 mmol of H2O2, in 1 mL of H2O for 3 h. Blue (conversion), red (sulfoxide) and green (sulfone). Conversion calculated based on MPS. | ||
Several non-polar and polar solvents were investigated for their effect on the selectivity and yield towards the sulfoxide product through the model reaction. Among them, H2O/CH2Cl2 (87%) and H2O (86%) gave better results for selective sulfoxide synthesis. However, due to the fact that water is a nontoxic, available and therefore greener solvent and also gave a slightly higher product yield, water was selected as the optimized solvent towards selective synthesis of sulfoxides. In the model reaction, the turnover number (TON) of the reaction system based on moles of sulfoxide as the main product per moles of “Fe3+” as the active center of the catalyst was 88.3. Under the same conditions, the turn over frequency (TOF) was 2.94 (h−1).
Thus, after appropriate studies for the optimization of the selective oxidation of MPS to the corresponding sulfoxide, the system was extended to other derivatives of sulfides and the results obtained from the derivatives are depicted in Table 1. Table 1 shows that the presence of electron-withdrawing groups on the aromatic ring generally caused a decrease in the reaction yield. Comparably, electron-donating substituents generally provided excellent yields of the products; however, slightly extended times of reaction were needed for reaction completion.
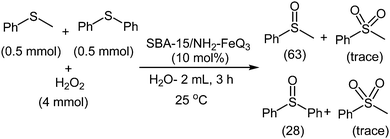
The activity and selectivity of SBA-15/NH2-FeQ3 in the oxidation of sulfides was also studied under the optimized conditions. When we inspected the reaction of a mixture of MPS and diphenyl sulfide under the reaction conditions, the yields of methyl phenyl sulfoxide and diphenyl sulfoxide were 64 and 28%, respectively (Scheme 3).
The ability to recycle the SBA-15/NH2-FeQ3 was also studied. When the reaction was complete, the catalyst was easily filtered from the reaction mixture. As illustrated in Fig. 10a, the catalyst could be reused over at least 10 runs without significant loss of activity. After the 10th run, the yield of sulfoxide decreased to 55%. This indicated that the FeQ3 immobilized SBA-15/NH2 was robust and recyclable. According to atomic absorption spectroscopy, no Fe was detected in the reaction solution until the 10th run, and elemental mapping analysis confirmed the good stability of the catalyst under the investigated reaction conditions even after the 10th run (Fig. 10b).
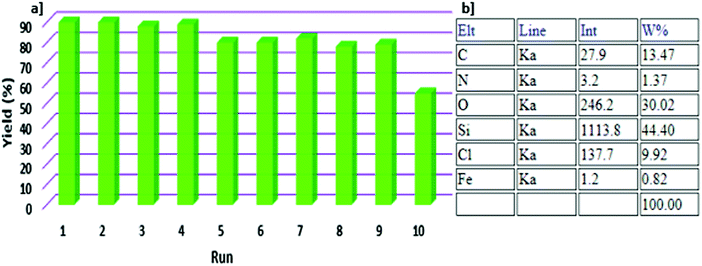 | ||
| Fig. 10 Recyclability of SBA-15/NH2-FeQ3 (a) and the corresponding elemental mapping data after the 10th run (b). | ||
In comparison to previously reported methods, our method is milder, greener from the viewpoint of the oxidant, support, and metal, and cost effective with higher conversion and selectivity towards the production of sulfoxides. This comparison clearly exhibits how well our proposed catalyst plays the role of a catalyst (Table 2).
| Entry | Catalyst | Oxidant | Solvent | T (°C)/t (h) | Yieldref. (%) |
|---|---|---|---|---|---|
| a 1-Methyl-1-phenylethyl hydroperoxide. | |||||
| 1 | Na2WO4 | H2O2 (2.5 eq.) | H2O | 50/1 | 9714 |
| 2 | Ti4+ | TBHP | CCl4 | 25/9 | 2815 |
| 3 | — | Oxone (1.3 eq.) | MeOH | 0/0.03 | 8417 |
| 4 | [WO4][SiO2/PrNH3]2 | H2O2 | DCM/MeOH | 25/0.5 | 8218 |
| 5 | VO(salen) | CHPa | DCM | −20/720 | 7919 |
| 6 | SBA-15/NH2-FeQ3 | H2O2 | H2O | 25/3 | 87this work |
A possible mechanism for sulfide oxidation using H2O2 over the SBA-15/NH2-FeQ3 catalyst is proposed in Scheme 4. Based on literature reports,33–37 we propose that SBA-15/NH2-FeQ3 easily reacts with H2O2 to form an FeIII–OOH adduct with a pendant OOH through the opening of its longest Fe–O bond. Then, the FeIII–OOH adduct may undergo homolytic cleavage of its O–OH bond to generate [FeIII–O]˙ and OH˙ radicals, and MPS can be oxidized to the corresponding sulfoxide (or sulfone in repeat steps) by the OH˙ radicals.
4. Conclusion
In summary, we have presented a potent supported tris(8-quinolinolato)iron-based SBA-15/NH2-FeQ3 mesoporous material that shows extraordinary catalytic activity and recyclability in green and selective sulfide oxidation. This separable solid porous catalyst can be prepared by a very simple procedure using inexpensive and commercially available precursors, and the catalyst can be reused for ten cycles without any loss in its activity, or any change in the composition of the catalyst. Thus, this procedure serves as a valuable alternative in the selective synthesis of sulfoxides.Conflicts of interest
There are no conflicts to declare.References
- X. Liu, P. Wang, Y. Yang, P. Wang and Q. Yang, Chem. – Asian J., 2010, 5, 1232–1239 CrossRef CAS PubMed.
- M. B. Gawande, V. D. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522–5551 RSC.
- X.-S. Wang, S. Ma, D. Sun, S. Parkin and H.-C. Zhou, J. Am. Chem. Soc., 2006, 128, 16474–16475 CrossRef CAS PubMed.
- X. Zhang, L. Jing, F. Chang, S. Chen, H. Quan Yang and Q. Yang, Chem. Commun., 2017, 53, 7780–7783 RSC.
- M. Heidarizadeh, E. Doustkhah, S. Rostamnia, P. F. Rezaei, F. D. Harzevili and B. Zeynizadeh, Int. J. Biol. Macromol., 2017, 101, 696–702 CrossRef CAS PubMed.
- E. Doustkhah, S. Rostamnia, B. Gholipour, B. Zeynizadeh, A. Baghban and R. Luque, Mol. Catal., 2017, 434, 7–15 CrossRef CAS.
- A. Baghban, M. Heidarizadeh, E. Doustkhah, S. Rostamnia and P. F. Rezaei, Int. J. Biol. Macromol., 2017, 103, 1194–1200 CrossRef CAS PubMed.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403–1419 CrossRef CAS.
- R. Liu, D. Wu, X. Feng and K. Müllen, Angew. Chem., 2010, 122, 2619–2623 CrossRef.
- D. E. De Vos, M. Dams, B. F. Sels and P. A. Jacobs, Chem. Rev., 2002, 102, 3615–3640 CrossRef CAS PubMed.
- M. Mureseanu, A. Reiss, I. Stefanescu, E. David, V. Parvulescu, G. Renard and V. Hulea, Chemosphere, 2008, 73, 1499–1504 CrossRef CAS PubMed.
- X.-F. Wu, Tetrahedron Lett., 2012, 53, 4328–4331 CrossRef CAS.
- N. J. Leonard and C. R. Johnson, J. Org. Chem., 1962, 27, 282–284 CrossRef CAS.
- K. Sato, M. Hyodo, M. Aoki, X.-Q. Zheng and R. Noyori, Tetrahedron, 2001, 57, 2469–2476 CrossRef CAS.
- N. Komatsu, M. Hashizume, T. Sugita and S. Uemura, J. Org. Chem., 1993, 58, 4529–4533 CrossRef CAS.
- K. Kaczorowska, Z. Kolarska, K. Mitka and P. Kowalski, Tetrahedron, 2005, 61, 8315–8327 CrossRef CAS.
- B. M. Trost and D. P. Curran, Tetrahedron Lett., 1981, 22, 1287–1290 CrossRef CAS.
- B. Karimi, M. Ghoreishi-Nezhad and J. H. Clark, Org. Lett., 2005, 7, 625–628 CrossRef CAS PubMed.
- K. Nakajima, K. Kojima, M. Kojima and J. Fujita, Bull. Chem. Soc. Jpn., 1990, 63, 2620–2630 CrossRef CAS.
- C. M. Gordon, Appl. Catal., A, 2001, 222, 101–117 CrossRef CAS.
- S. Rostamnia, RSC Adv., 2015, 5, 97044–97065 RSC.
- S. Rostamnia, E. Doustkhah, H. Golchin-Hosseini, B. Zeynizadeh, H. Xin and R. Luque, Catal. Sci. Technol., 2016, 6, 4124–4133 CAS.
- S. Rostamnia, E. Doustkhah, K. Bahrami and S. Amini, J. Mol. Liq., 2015, 207, 334–337 CrossRef CAS.
- S. Rostamnia, E. Doustkhah, R. Bulgar and B. Zeynizadeh, Microporous Mesoporous Mater., 2016, 225, 272–279 CrossRef CAS.
- S. Rostamnia, C. R. Chim., 2013, 16, 1042–1046 CrossRef CAS.
- E. Doustkhah and S. Rostamnia, J. Colloid Interface Sci., 2016, 478, 280–287 CrossRef CAS PubMed.
- S. Rostamnia and E. Doustkhah, Green Chemistry: Synthesis of Bioactive Heterocycles, Springer, 2014, pp. 253–275 Search PubMed.
- S. Rostamnia, B. Gholipour and H. G. Hosseini, Process Saf. Environ. Prot., 2016, 100, 74–79 CrossRef CAS.
- S. Rostamnia, E. Doustkhah, A. Baghban and B. Zeynizadeh, J. Appl. Polym. Sci., 2016, 133, 43190 CrossRef.
- S. Rostamnia, H. Alamgholiloo, M. Jafari, R. Rookhosh and A. R. Abbasi, Appl. Organomet. Chem., 2016, 11, 954–958 CrossRef.
- S. Rostamnia and T. Rahmani, Appl. Organomet. Chem., 2015, 29, 471–474 CrossRef CAS.
- E. Doustkhah, S. Rostamnia, H. G. Hossieni and R. Luque, ChemistrySelect, 2017, 2, 329–334 CrossRef CAS.
- Q. Yan, Y. C. Fang, Y. X. Jia and X. H. Duan, New J. Chem., 2017, 41, 2372–2377 RSC.
- S. Tanaka, Y. Kon, T. Nakashima and K. Sato, RSC Adv., 2014, 4, 37674–37678 RSC.
- S. Zhong, Y. Tan, Z. Fu, Q. Xie, F. Xie, X. Zhou, Z. Ye, G. Peng and D. Yin, J. Catal., 2008, 256, 154–158 CrossRef CAS.
- Y. Wang, Z. Fu, X. Wen, C. Rong, W. Wu, C. Zhang, J. Deng, B. Dai, S. R. Kirk and D. Yin, J. Mol. Catal. A: Chem., 2014, 383, 46–52 CrossRef.
- Y. Wang, X. Wen, C. Rong, S. Tang, W. Wu, C. Zhang, Y. Liu and Z. Fu, J. Mol. Catal. A: Chem., 2016, 411, 103–109 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |