Construction of the Bi2WO6/Bi4V2O11 heterojunction for highly efficient visible-light-driven photocatalytic reduction of Cr(VI)†
Chol-Nam
Ri
 *ab,
Kim
Song-Gol
a,
Jong
Ju-Yong
bc,
Sung-Nam
Pak
bc,
Song-Chon
Ri
a and
Jong-Hwa
Ri
a
*ab,
Kim
Song-Gol
a,
Jong
Ju-Yong
bc,
Sung-Nam
Pak
bc,
Song-Chon
Ri
a and
Jong-Hwa
Ri
a
aInstitute for Electronic Materials, Kim Il Sung University, Pyongyang, Democratic People's Republic of Korea
bSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150001, China. E-mail: rcn906@yahoo.com
cDepartment of Energy Science, Kim Il Sung University, Pyongyang, Democratic People's Republic of Korea
First published on 27th November 2017
Abstract
Herein, a Bi2WO6/Bi4V2O11 heterostructured photocatalyst was fabricated through a facile one-pot solvothermal method. In this heterojunction system, highly homogeneous-dispersed Bi4V2O11 nanocrystals were anchored onto Bi2WO6 nanoflakes, endowing the heterojunction with nanosized interfacial contact. Because of the favorable interfacial contact and band alignment, photoinduced charge carrier transfer is facilitated in the Bi2WO6/Bi4V2O11 heterojunction, which can be verified by photocurrent and photoluminescence measurements. In contrast with pristine Bi2WO6 and Bi4V2O11 samples, the Bi2WO6/Bi4V2O11 heterostructured photocatalyst displays drastically improved photocatalytic Cr(VI) reduction activity under visible-light irradiation. More significantly, the as-fabricated heterojunction possesses superior activity for Cr(VI) relative to the counterpart obtained by mechanical mixing, indicating that the interfacial contact plays a determinant role in promoting charge carrier transfer. Moreover, Bi2WO6/Bi4V2O11 heterojunctions exhibited good cycling stability even after 5 cycles. A plausible photocatalytic Cr(VI) reduction reaction mechanism over the Bi2WO6/Bi4V2O11 heterojunction is proposed on the basis of band alignment. This work reports a high-efficiency photocatalyst for Cr(VI) and might shed light on the decisive factor for optimizing the heterostructure.
1. Introduction
Nowadays, energy shortage and environment pollution are two prominent global issues. Photocatalysis as a ‘‘green’’ technology, possessing bright potential for the development of renewable energy and the elimination of contaminants, has attracted substantial attention in the past few decades. The fabrication of visible-light-driven photocatalysts with outstanding catalytic activity and stability has drawn considerable attention.1–3In the past few decades, Bi2WO6 with a layered structure consisting of alternating (Bi2O2)2+ layers and (WO4)2− octahedral layers has been considered as a promising photocatalyst, which possesses visible-light harvesting ability for catalysis reactions because of its narrow band gap (2.6–2.8 eV).4–6 Unfortunately, pristine Bi2WO6 can only harvest light with a wavelength less than approximately 450 nm, which takes up a tiny portion of solar light.7,8 Besides a rapid recombination of photoinduced electron–hole pairs, sluggish charge transport in pristine Bi2WO6 will greatly restrict its expectable photocatalytic activity.9–11 In order to overcome the obstacles of limited light absorption and a rapid recombination of charge carriers, many strategies have been developed, such as doping,7 controlling morphology12 and nanostructuring.13
Among them, the method of constructing heterojunctions stands out since it can efficiently boost charge carrier separation and transfer stemming from the as-introduced built-in electric field at the heterostructured interfaces.14–16 For example, Liu's group reported the Ag2O modified Bi2WO6 heterojunction photocatalysts synthesized by the solution precipitation strategy and the results showed that the Ag2O/Bi2WO6 composite photocatalyst exhibited obviously enhanced photocatalytic activity compared with pure Ag2O and Bi2WO6 for the degradation of organic contaminants.17 To date, different types of Bi2WO6-based composite photocatalysts have been fabricated with increased photocatalytic efficiency, which can be roughly classified into the composites of Bi2WO6 with oxides such as Bi2O3/Bi2WO6,18 Co3O4/Bi2WO6,19 WO3/Bi2WO6,20 ZnO/Bi2WO6,21 TiO2/Bi2WO6,22 the composites of Bi2WO6 with sulphides such as Bi2S3/Bi2WO6,23 Bi2WO6/CdS,24 the composites of Bi2WO6 with BiOX such as BiOCl/Bi2WO6,25 BiOBr/Bi2WO6,26 BiOI/Bi2WO6,27 the composites of Bi2WO6 with carbonaceous materials such as C3N4/Bi2WO6,28,29 Ag2CO3/Bi2WO6,30 Bi2O2CO3/Bi2WO6,31 the Bi-based compounds such as Bi2WO6/BiVO432 and Bi2MoO6/Bi2WO6,33 and the composites of Bi2WO6 with noble metals such as Ag loaded Bi2WO6,34etc.
Very recently, Bi4V2O11 with a peculiar layered structure which has intrinsic oxygen vacancies in perovskite (VO3.5□0.5)2− (□ represents intrinsic oxygen vacancies) slabs sandwiched between (Bi2O2)2+ layers is considered as an excellent photocatalyst for oxygen evolution and water purification.35–39 Because of the narrow band gap (∼2.1 eV) and excellent charge carrier transport stemming from its unique crystalline structure, Bi4V2O11 has been employed to construct heterojunction photocatalysts. Lv and co-workers fabricated the BiVO4/Bi4V2O11 heterojunction nanofibres via a facile electrospinning process and evaluated their photocatalytic activity for the degradation of RhB. The results indicated that the photocatalytic performance of BiVO4/Bi4V2O11 heterojunction nanofibres was markedly enhanced compared with that of pure BiVO4 nanofibres.40 Liu et al. reported the preparation of Bi24O31Br10/Bi4V2O11 heterojunction photocatalysts using a facile one-pot solvothermal method and the photocatalysis experimental results revealed that Bi24O31Br10/Bi4V2O11 heterojunctions exhibited high photocatalytic activity towards the degradation of RhB compared with bare Bi24O31Br10 and Bi4V2O11.41
All of the above heterojunctions realize dramatically enhanced photocatalytic activity due to the effective separation and transfer of photoinduced charge carriers. Nevertheless, to the best of our knowledge, the construction of the Bi2WO6/Bi4V2O11 heterojunction has not been reported, which might promote the photocatalytic properties of Bi2WO6 dramatically.
In this study, the Bi2WO6/Bi4V2O11 heterojunction was fabricated using a one-pot solvothermal method. This heterojunction is constructed from Bi2WO6 nanoflakes and anchored Bi4V2O11 nanocrystals, which achieves a nanosized interfacial contact. The photocatalytic performances of the as-fabricated pristine Bi2WO6, Bi4V2O11 and different Bi2WO6/Bi4V2O11 heterojunctions were evaluated by photocatalytic Cr(VI) reduction under visible-light illumination, and the photocatalytic experimental results indicated that the Bi2WO6/Bi4V2O11 heterojunction exhibited a higher photocatalytic activity than pristine Bi2WO6 and Bi4V2O11. Finally, the enhanced photocatalytic mechanism of the Bi2WO6/Bi4V2O11 heterojunction has also been discussed.
2. Experimental
2.1 Photocatalysts preparation
All chemical reagents were received from Aladdin Chemical Co., Ltd, and used without further purification.2.0 mmol of Bi(NO3)3·5H2O was dissolved in 16 mL of ethylene glycol (EG) in a water bath at 80 °C under magnetic stirring. Then, appropriate stoichiometric amounts of Na2WO4·2H2O and NH4VO3 were added to the above Bi(NO3)3 solution, which was subsequently continuously stirred for 20 min in a water bath at 80 °C to obtain a transparent solution. In order to fixed the pH value of 8, 2 mL of 2 M NaOH solution was slowly dropped into the above solution and subsequently 16 mL of absolute ethanol was added. Then, the above solution was poured into a 50 mL Teflon-lined stainless autoclave, which was subsequently sealed and maintained at 160 °C for 16 h in an oven. After the reactor cooled down naturally to room temperature, the as-obtained product was centrifuged using water and absolute ethanol three times, respectively. Finally, the photocatalyst was obtained after drying treatment at 80 °C in air for 4 h. According to the abovementioned method, Bi2WO6/Bi4V2O11 heterojunction photocatalysts with different mole amounts of Na2WO4·2H2O and NH4VO3 at 0.8 mmol and 0.2 mmol, respectively (denoted as BWV-82), 0.6 mmol and 0.4 mmol, respectively (denoted as BWV-64), 0.4 mmol and 0.6 mmol, respectively (denoted as BWV-46) were prepared, in which the mole amount of Bi(NO3)3·5H2O was fixed as 2 mmol, and the total mole amounts of Na2WO4·2H2O and NH4VO3 are 1 mmol. In addition, pristine Bi2WO6, Bi4V2O11 and mechanically mixed Bi2WO6/Bi4V2O11 (W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 8
V = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, denoted as BWV-82-MM) were also fabricated for the purpose of comparison.
2, denoted as BWV-82-MM) were also fabricated for the purpose of comparison.
2.2 Characterizations
The crystalline phases of the as-fabricated samples were analyzed by X-ray diffraction (XRD) on a Rigaku D/max-2000 diffractometer with Cu Kα radiation (λ = 1.5406 Å) in the range of 2θ = 20°–90° at a scanning rate of 4 °C min−1 with a scan width of 0.02°. The morphology of the samples was observed using a field emission scanning electron microscope (FE-SEM, HELIOS NanoLab 600i) with an accelerating voltage of 20 kV. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) analyses were carried out on a JEM-2100 transmission electron microscope at an accelerating voltage of 200 kV. X-Ray photoelectron spectroscopy (XPS) was conducted using a Thermo Scientific ESCALAB 250Xi X-ray photoelectron spectrometer coupled with a pass energy of 20.00 eV and an Al Kα excitation source (1486.6 eV). UV-vis diffuse reflectance spectra (DRS) were recorded using a spectrophotometer (HITACHI UH-4150) with BaSO4 as the reflectance standard. The photoluminescence (PL) spectra were recorded on HORIBA FluoroMax-4.2.3 Photocatalytic activity and photoelectrochemical measurements
The photocatalytic performance of the as-fabricated samples was evaluated by the reduction of Cr(VI) with citric acid as a hole scavenger under visible-light illumination using a 300 W Xe lamp (Trusttech PLS-SXE 300, Beijing) with a UV cutoff filter (λ ≥ 400 nm). In a typical photocatalytic measurement process, 0.05 g of the as-fabricated sample was added to 100 mL of Cr(VI) solution (10 mg L−1, which was based on Cr in a dilute K2Cr2O7 solution). The photocatalyst was dispersed in the Cr(VI) solution under ultrasonic treatment for 10 min, and the mixed solution was magnetically stirred in the dark for 30 min to reach adsorption–desorption equilibrium between the photocatalyst and the degrading pollutants, then adding 0.05 g of citric acid to the reactor before irradiation. When the photodegradation experiment began, 4 mL of the solution was collected from the suspension at fixed-time intervals, subsequently centrifuged at 104 rpm for 5 min to remove catalyst powders, and the Cr(VI) concentration was determined at 352 nm by using the HITACHI UH-5300 UV-vis spectrometer.The photoelectrochemical measurements were carried out on a CHI604C electrochemical working station using a standard three-compartment cell with Bi4V2O11, Bi2WO6 and Bi2WO6/Bi4V2O11 samples coated at FTO glass as the working electrodes, a piece of Pt sheet as a counter electrode and standard Ag/AgCl in saturated KCl as a reference electrode, a 0.5 M Na2SO4 aqueous solution as an electrolyte. Conversion between the measured potential (vs. Ag/AgCl) and NHE is achieved through the following equation.42
| ENHE = EAg/AgCl + 0.1976 (25 °C) |
The light source employed was a 300 W Xe lamp. The Mott–Schottky measurements were carried out at a frequency of 100 Hz with 10 mV amplitude.
3. Results and discussion
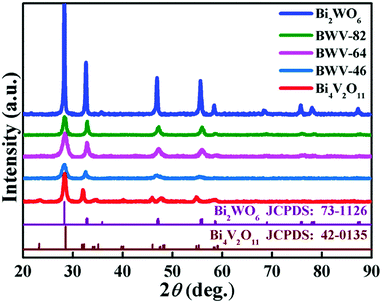
Fig. 1 shows the X-ray diffraction (XRD) patterns of the as-prepared pristine Bi2WO6, Bi4V2O11 and Bi2WO6/Bi4V2O11 heterojunctions. The diffraction peaks of the pristine Bi2WO6 and Bi4V2O11 samples can be well indexed to the orthorhombic phase Bi2WO6 (JCPDS No. 73-1126) and the orthorhombic phase Bi4V2O11 (JCPDS No. 42-0135), respectively. The XRD patterns of Bi2WO6/Bi4V2O11 heterostructured samples are similar to those of pristine Bi2WO6, while no characteristic peak belonging to Bi4V2O11 could be observed. The absence of XRD peaks of Bi4V2O11 might be due to their tiny size and uniform dispersion on the surface of Bi2WO6,43–45 which is displayed as below. The morphology and microstructure of samples are investigated through scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Pristine Bi2WO6 (Fig. 2a) exhibits irregular flake-like morphologies with a thickness of about 30 nm. In contrast, pure Bi4V2O11 (Fig. 2b) displays uniform and monodispersed hierarchical flower-like microspheres with a diameter of approximately 1 μm. When the heterojunction is constructed by Bi2WO6 with Bi4V2O11, a representative sample of BWV-82 possesses irregular nanoflake morphology, whose size is smaller than that of pristine Bi2WO6 (Fig. 2c). The TEM image of BWV-82 (Fig. 2d and Fig. S1, ESI†) further illustrates that the nanoflakes are ca. 5 nm in thickness and 100 nm in length. Interestingly, as observed from the magnified TEM image (Fig. 2e) of Fig. 2d, nanocrystals with a size of approximately 6 nm are homogeneously dispersed and tightly adhered to the surface of the nanoflakes.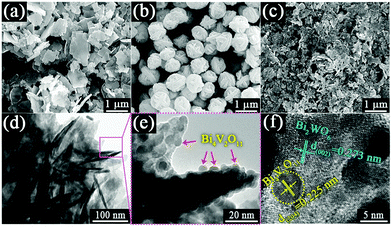 | ||
| Fig. 2 FESEM images of (a) Bi2WO6, (b) Bi4V2O11 and (c) BWV-82; (d), (e) TEM images and (f) HRTEM image of BWV-82. | ||
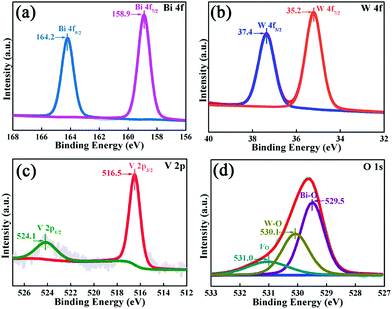
As vividly elucidated in a high-resolution TEM (HRTEM) image (Fig. 2f), the interplanar spacing of the nanoflakes is 0.273 nm which could correspond to the (002) plane of the orthorhombic Bi2WO6, while the interplanar spacing of the as-anchored nanocrystals is 0.225 nm, assigned to the (204) plane of orthorhombic Bi4V2O11. Thus, the Bi2WO6/Bi4V2O11 heterojunction is successfully constructed by Bi2WO6 nanoflakes with the as-anchored Bi4V2O11 nanocrystals. Moreover, attributed to this unique configuration, nanosized interfacial contact is also achieved due to the tiny size of Bi4V2O11 nanocrystals. In addition, in the morphology of the BWV-82 heterojunction, no flower-like hierarchical structure of Bi4V2O11 microspheres was observed and Bi4V2O11 became tiny nanocrystals, which could be attributed to the restricted crystal growth of Bi4V2O11 due to the confined space effect of two-dimensional Bi2WO6 nanoflakes.46 In addition, the SEM images of Bi2WO6/Bi4V2O11 heterojunctions with various mole amounts of Na2WO4·2H2O and NH4VO3 are shown in the ESI,† Fig. S2. The optical absorption properties of as-prepared photocatalysts are analyzed by UV-vis diffuse reflection spectroscopy (DRS). From the observation of UV-vis DRS spectra (Fig. 3), it can be clearly seen that the absorption edges of pristine Bi2WO6 and Bi4V2O11 are located at ca. 440 nm and 612 nm, respectively. In contrast, the absorption spectra of Bi2WO6/Bi4V2O11 heterojunctions exhibited the combined absorption properties of pristine Bi2WO6 and Bi4V2O11. This result further provided the evidence for constructing the heterojunction between Bi2WO6 and Bi4V2O11. Moreover, the Bi2WO6/Bi4V2O11 heterojunctions exhibit an enhanced light absorption ability and a red shift of the absorption edge compared to pristine Bi2WO6. It indicates that the introduction of Bi4V2O11 is beneficial for light-harvesting of Bi2WO6/Bi4V2O11 heterojunctions. With the purpose of demonstrating the surface electronic states and chemical compositions of the Bi2WO6/Bi4V2O11 heterojunction photocatalysts, X-ray photoelectron spectroscopy (XPS) is carried out. Fig. 4 shows the XPS spectra of the as-prepared BWV-82 heterojunction photocatalyst. The survey spectra and C ls spectra of BWV-82 are shown in Fig. S3 (ESI†). The peak positions in all of the XPS spectra are calibrated using those of C 1s (284.6 eV) spectra. As displayed in Bi 4f spectra (Fig. 4a), two photoelectron peaks located at 164.2 and 158.9 eV are assigned to Bi 4f5/2 and Bi 4f7/2, respectively, which corresponds to Bi3+.47 In W 4f XPS spectra (Fig. 4b), the binding energies for W 4f5/2 and W 4f7/2 are 37.4 and 35.2 eV, respectively, which are assigned to W6+ in Bi2WO6.48 For V 2p peaks (Fig. 4c), two peaks located at 524.1 and 516.5 eV are assigned to V 2p1/2 and V 2p3/2, respectively, which belong to V5+ in Bi4V2O11.37,41 Moreover, Fig. 4d shows the O 1s XPS, which can be divided into three peaks. The binding energies of 529.5, 530.1 and 531.0 eV could be assigned to the lattice oxygen in Bi–O,49 the lattice oxygen in W–O,25 and intrinsic oxygen vacancies (VO) in Bi4V2O11,50,51 respectively. The XPS results further demonstrate the presence of Bi2WO6 and Bi4V2O11 species in BWV-82. The photocatalytic activity of the as-prepared photocatalysts is evaluated by photocatalytic reduction of a toxic heavy metal ion Cr(VI) in an aqueous solution. As shown in Fig. 5a, the Bi2WO6/Bi4V2O11 heterojunction photocatalysts exhibit higher photocatalytic activity for the reduction of Cr(VI) relative to pristine Bi2WO6 and Bi4V2O11, indicating the superiority of the constructed heterojunction.
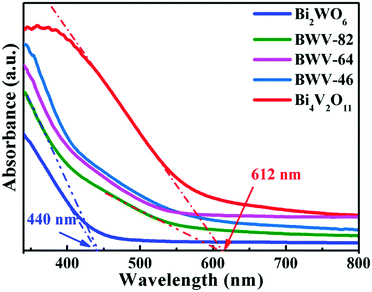 | ||
| Fig. 3 UV-vis diffuse reflectance spectra (DRS) of pristine Bi2WO6, Bi4V2O11 and Bi2WO6/Bi4V2O11 heterojunctions. | ||
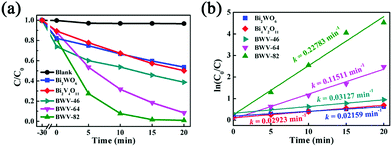 | ||
| Fig. 5 (a) Photocatalytic reduction curves of Cr(VI) aqueous solution in the presence of various catalysts under visible-light illumination, (b) photocatalytic reduction kinetics of Cr(VI). | ||
In particular, the BWV-82 sample possesses the most standout photocatalytic properties among the samples and almost completely reduces Cr(VI) to Cr(III) within 20 min. Furthermore, the reaction rate of the photocatalytic reduction of Cr(VI) is evaluated by the apparent pseudo-first-order model according to the Langmuir–Hinshelwood (L–H) kinetics model.52
| ln(C0/C) = kt |
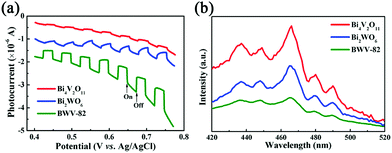
However, even when citric acid was not added, the photocatalytic performance of BWV-82-NS was found to be superior to that of pristine Bi2WO6 and Bi4V2O11. With the aim to confirm the viewpoint, transient photocurrent analysis was conducted, which is widely recognized as a typical method used to evaluate the separation effect of photoinduced charge carriers for a photocatalyst.53Fig. 6a presents the photocurrent intensities of pristine Bi2WO6, Bi4V2O11 and the Bi2WO6/Bi4V2O11 heterojunction (BWV-82) from 0.4 to 0.8 eV (vs. Ag/AgCl) under visible-light illumination. Obviously, the BWV-82 sample exhibits a significantly enhanced photocurrent response, and approximately 4 and 10-fold promotions are realized in sharp contrast with pristine Bi2WO6 and Bi4V2O11, respectively. It reveals that BWV-82 possesses a much higher separation and transport efficiency of photoinduced electrons and holes. In order to further verify the enhanced separation efficiency of photoinduced charge carriers in BWV-82, photoluminescence (PL) spectra analysis is carried out, since recording the PL spectra is an effective method for monitoring the recombination efficiency of photoinduced electrons and holes. Generally, there is a strong correlation between the photocatalytic activity and the PL intensity, that is, a lower recombination rate leads to a lower PL intensity and a higher photocatalytic activity.54 As illustrated in the PL spectra of the pristine Bi2WO6, Bi4V2O11 and BWV-82 excited at a wavelength of 320 nm (Fig. 6b), BWV-82 displays a lower PL emission intensity than those of pristine Bi2WO6 and Bi4V2O11, indicating that the recombination rate of photoinduced electron–hole pairs is effectively hindered in the Bi2WO6/Bi4V2O11 heterojunction (BWV-82). This result is quite consistent with the abovementioned transient photocurrent response analysis. Thereby, it can be concluded that the construction of a heterojunction by combining Bi2WO6 nanoflakes and Bi4V2O11 nanocrystals is extremely beneficial to the separation and transfer of photoinduced charge carriers, thus highly enhancing the photocatalytic activity.46 Attributed to the unique configuration, nanosized interfacial contact is achieved in the as-constructed Bi2WO6/Bi4V2O11 heterojunction, which might play a determinant role in promoting the separation and transfer of photoinduced charge carriers. In order to confirm the above argument, the photocatalytic Cr(VI) reduction experiment over the mechanically mixed Bi2WO6/Bi4V2O11 (W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 8
V = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, denoted as BWV-82-MM) is also performed and illustrated in Fig. S4 (ESI†). The mechanically mixed BWV-82-MM sample shows much lower photocatalytic activity than the BWV-82 heterojunction prepared by a one-pot solvothermal approach, which only reaches 50% reduction rate of Cr(VI) in 20 min under visible-light irradiation. This could result from the significant difference in the heterostructured interfaces of the two samples. Through the simple mechanical mixing process, only loose and diffuse interfaces are introduced due to the large size of Bi4V2O11 microspheres instead of nanocrystals with little contact retardation. Such an insufficient contact interface could suppress the separation and transfer of photoinduced charge carriers. However, the as-constructed BWV-82 heterojunction photocatalyst possesses interfaces with nanosized close contact, which could promote interfacial carrier transfer, resulting in drastically promoted photocatalytic Cr(VI) reduction performance. This result proves the superiority of the as-constructed heterojunction and implies that the intimate interface between Bi2WO6 and Bi4V2O11 plays a significant role in enhancing the photocatalytic activity.
2, denoted as BWV-82-MM) is also performed and illustrated in Fig. S4 (ESI†). The mechanically mixed BWV-82-MM sample shows much lower photocatalytic activity than the BWV-82 heterojunction prepared by a one-pot solvothermal approach, which only reaches 50% reduction rate of Cr(VI) in 20 min under visible-light irradiation. This could result from the significant difference in the heterostructured interfaces of the two samples. Through the simple mechanical mixing process, only loose and diffuse interfaces are introduced due to the large size of Bi4V2O11 microspheres instead of nanocrystals with little contact retardation. Such an insufficient contact interface could suppress the separation and transfer of photoinduced charge carriers. However, the as-constructed BWV-82 heterojunction photocatalyst possesses interfaces with nanosized close contact, which could promote interfacial carrier transfer, resulting in drastically promoted photocatalytic Cr(VI) reduction performance. This result proves the superiority of the as-constructed heterojunction and implies that the intimate interface between Bi2WO6 and Bi4V2O11 plays a significant role in enhancing the photocatalytic activity.
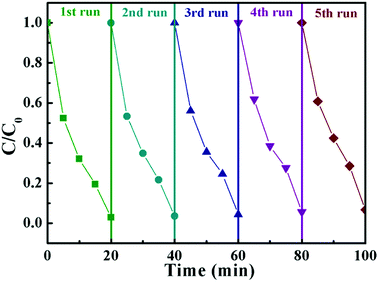
From the point of view of practical application, cycling stability of photocatalysts is one very important factor for evaluating photocatalytic performances. The circulation runs for the photocatalytic Cr(VI) reduction with the BWV-82 heterojunction are carried out to evaluate its cycling stability. Prior to the next cycle experiment, the previously used photocatalyst is subjected to ultrasonic cleaning with distilled water and drying at 60 °C. Fig. 7 displays the results of the recycling experiment with 5 cycles. After 5 cycles, the photocatalytic Cr(VI) reduction rate still remains above 90%, which indicates that the as-fabricated BWV-82 heterojunction photocatalyst possesses good cycling stability. With the aim of understanding the mechanism of the enhanced photocatalytic activity in the Bi2WO6/Bi4V2O11 heterojunction, the energy band structure is investigated by determining the optical band gaps and flat band potentials of pristine Bi2WO6 and Bi4V2O11. The band gap of the semiconductor can be calculated by the Kubelka–Munk function:55
| αhν = A(hν − Eg)n/2 |
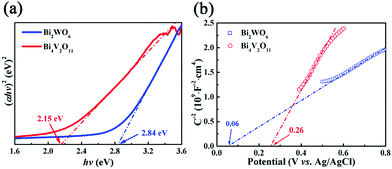 | ||
| Fig. 8 (a) Plots of (αhν)2vs. hν of pristine Bi2WO6 and Bi4V2O11 samples, (b) Mott–Schottky plots of pristine Bi2WO6 and Bi4V2O11. | ||
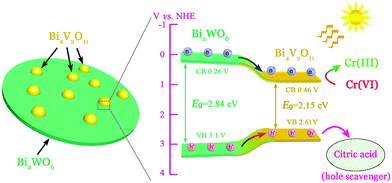 | ||
| Fig. 9 Schematic illustrations of the photoinduced charge carrier separation and the photocatalytic Cr(VI) reduction reaction mechanism over the Bi2WO6/Bi4V2O11 heterojunctions. | ||
4. Conclusions
In summary, a novel visible-light responsive Bi2WO6/Bi4V2O11 heterojunction photocatalyst with nanosized interfacial contact has been successfully fabricated using a facile one-pot solvothermal method. The Bi2WO6/Bi4V2O11 heterojunctions show excellent photocatalytic Cr(VI) reduction activity under visible-light illumination. In particular, the sample of the BWV-82 heterojunction exhibits the highest photocatalytic Cr(VI) reduction efficiency which is approximately 10 times higher than those of pristine Bi2WO6 and Bi4V2O11. In addition, the Bi2WO6/Bi4V2O11 heterojunction also displays enhanced transient photocurrent response and lower PL intensity, which indicated that the photoinduced charge carriers are more effectively separated and transferred to the Bi2WO6/Bi4V2O11 heterojunctions.The remarkably enhanced photocatalytic activity and the separation effect of the charge carriers are attributed to the formation of a heterojunction with a nanosized interfacial contact between Bi2WO6 nanoflakes and Bi4V2O11 nanocrystals. Moreover, Bi2WO6/Bi4V2O11 heterojunctions exhibit good cycling stability even after 5 cycles. This work may be further extended to the research and development of a novel semiconductor heterojunction containing a new kind of Bi4V2O11 photocatalyst for water purification and related applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (21471040).References
- Y. Q. Qu and X. F. Duan, Chem. Soc. Rev., 2013, 42, 2568 RSC.
- M. Z. Ge, C. Y. Cao, J. Y. Huang, S. H. Li, Z. Chen, K. Q. Zhang, S. S. Al-Deyab and Y. K. Lai, J. Mater. Chem. A, 2016, 4, 6772 CAS.
- X. Li, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603 RSC.
- A. Etogo, R. Liu, J. B. Ren, L. W. Qi, C. C. Zheng, J. Q. Ning, Y. J. Zhong and Y. Hu, J. Mater. Chem. A, 2016, 4, 13242 CAS.
- Y. H. Liang, S. L. Lin, L. Liu, J. S. Hu and W. Q. Cui, Appl. Catal., B, 2015, 164, 192 CrossRef CAS.
- J. Tian, Y. H. Sang, G. W. Yu, H. D. Jiang, X. N. Mu and H. Liu, Adv. Mater., 2013, 25, 5075 CrossRef CAS PubMed.
- H. W. Huang, K. Liu, K. Chen, Y. L. Zhang, Y. H. Zhang and S. C. Wang, J. Phys. Chem. C, 2014, 118, 14379 CAS.
- Y. Zheng, K. L. Lv, X. F. Li, K. J. Deng, J. Sun, L. Q. Chen, L. Z. Cui and D. Y. Du, Chem. Eng. Technol., 2011, 34, 1630 CrossRef CAS.
- X. C. Song, W. T. Li, W. Z. Huang, H. Zhou, H. Y. Yin and Y. F. Zheng, J. Nanopart. Res., 2015, 17, 134 CrossRef.
- S. X. Yu, Y. H. Zhang, M. Li, X. Du and H. W. Huang, Appl. Surf. Sci., 2017, 391, 491 CrossRef CAS.
- H. L. Wang, S. J. Li, L. S. Zhang, Z. G. Chen, J. Q. Hu, R. J. Zou, K. B. Xu, G. S. Song, H. H. Zhao, J. M. Yang and J. S. Liu, CrystEngComm, 2013, 15, 9011 RSC.
- X. N. Li, R. K. Huang, Y. H. Hu, Y. J. Chen, W. J. Liu, R. S. Yuan and Z. H. Li, Inorg. Chem., 2012, 51, 6245 CrossRef CAS PubMed.
- Z. P. Nie, D. K. Ma, G. Y. Fang, W. Chen and S. M. Huang, J. Mater. Chem. A, 2016, 4, 2438 CAS.
- M. Y. Ye, Z. H. Zhao, Z. H. Hu, L. Q. Liu, H. M. Ji, Z. R. Shen and T. Y. Ma, Angew. Chem., Int. Ed., 2017, 56, 1 CrossRef.
- H. L. Wang, L. S. Zhang, Z. G. Chen, J. Q. Hu, S. J. Li, Z. H. Wang, J. S. Liu and X. C. Wang, Chem. Soc. Rev., 2014, 43, 5234 RSC.
- J. Tian, Z. H. Zhao, A. Kumar, R. I. Boughton and H. Liu, Chem. Soc. Rev., 2014, 43, 6920 RSC.
- C. M. Liu, J. W. Liu, G. Y. Zhang, J. B. Zhang, Q. S. Wu, Y. Y. Xu and Y. Q. Sun, RSC Adv., 2015, 5, 32333 RSC.
- M. Ge, Y. F. Li, L. Liu, Z. Zhou and W. Chen, J. Phys. Chem. C, 2011, 115, 5220 CAS.
- Q. Xiao, J. Zhang, C. Xiao and X. K. Tan, Catal. Commun., 2008, 9, 1247 CrossRef CAS.
- M. S. Gui, W. D. Zhang, Y. Q. Chang and Y. X. Yu, Chem. Eng. J., 2012, 197, 283 CrossRef CAS.
- Y. L. Min, K. Zhang, Y. C. Chen, Y. G. Zhang and W. Zhao, Sep. Purif. Technol., 2012, 92, 115 CrossRef CAS.
- Y. P. Zhang, L. F. Fei, X. D. Jiang, C. X. Pan and Y. Wang, J. Am. Ceram. Soc., 2011, 94, 4157 CrossRef CAS.
- A. Rauf, M. S. A. S. Shah, G. H. Choi, U. B. Humayoun, D. H. Yoon, J. W. Bae, J. H. Park, W. J. Kim and P. J. Yoo, ACS Sustainable Chem. Eng., 2015, 3, 2847 CrossRef CAS.
- Y. F. Liu, S. S. Liu, T. T. Wu, H. M. Lin and X. Zhang, J. Sol-Gel Sci. Technol., 2017, 83, 315 CrossRef CAS.
- W. J. Yang, B. Ma, W. C. Wang, Y. W. Wen, D. W. Zeng and B. Shan, Phys. Chem. Chem. Phys., 2013, 15, 19387 RSC.
- J. S. Hu, W. J. An, H. Wang, J. P. Geng, W. Q. Cui and Y. Zhan, RSC Adv., 2016, 6, 29554 RSC.
- H. Q. Li, Y. M. Cui and W. S. Hong, Appl. Surf. Sci., 2013, 264, 581 CrossRef CAS.
- Y. L. Tian, B. B. Chang, J. L. Lu, J. Fu, F. N. Xi and X. P. Dong, ACS Appl. Mater. Interfaces, 2013, 5, 7079 CAS.
- Y. J. Wang, X. J. Bai, C. S. Pan, J. He and Y. F. Zhu, J. Mater. Chem., 2012, 22, 11568 RSC.
- J. X. Bao, S. H. Guo, J. Y. Gao, T. Hu, L. Yang, C. H. Liu, J. H. Peng and C. Y. Jiang, RSC Adv., 2015, 5, 97195 RSC.
- X. W. Huang and H. F. Chen, Appl. Surf. Sci., 2013, 284, 843 CrossRef CAS.
- P. Ju, P. Wang, B. Li, H. Fan, S. Y. Ai, D. Zhang and Y. Wang, Chem. Eng. J., 2014, 236, 430 CrossRef CAS.
- F. J. Zhang, S. F. Zhu, F. Z. Xie, J. Zhang and Z. D. Meng, Sep. Purif. Technol., 2013, 113, 1 CrossRef CAS.
- D. J. Wang, G. L. Xue, Y. Z. Zhen, F. Fu and D. S. Li, J. Mater. Chem., 2012, 22, 4751 RSC.
- X. F. Chen, J. B. Liu, H. Wang, Y. L. Ding, Y. X. Sun and H. Yan, J. Mater. Chem. A, 2013, 1, 877 CAS.
- Z. Y. Jiang, Y. Y. Liu, M. M. Li, T. Jing, B. B. Huang, X. Y. Zhang, X. Y. Qin and Y. Dai, Sci. Rep., 2016, 6, 22727 CrossRef CAS PubMed.
- Y. T. Lu, Y. F. Pu, J. Wang, C. X. Qin, C. L. Chen and H. J. Seo, Appl. Surf. Sci., 2015, 347, 719 CrossRef CAS.
- S. Kumar and P. D. Sahare, Nano, 2013, 8, 1350007 CrossRef.
- H. Q. N. M. Alarique, E. S. Al-Aghbari, N. A. S. Al-Areqi, A. Al-Alas and K. A. S. Ghaleb, Am. J. Phys. Chem., 2014, 3, 102 CrossRef.
- C. D. Lv, G. Chen, J. X. Sun, Y. S. Zhou, S. Fan and C. M. Zhang, Appl. Catal., B, 2015, 179, 54 CrossRef CAS.
- Z. S. Liu, J. N. Niu, P. Z. Feng, Y. W. Sui and Y. B. Zhu, RSC Adv., 2014, 4, 43399 RSC.
- X. Yang, X. J. Lian, S. J. Liu, J. Tian, C. P. Jiang and G. Wang, Int. J. Electrochem. Sci., 2013, 8, 3721 CAS.
- R. Dadigala, B. R. Gangapuram, R. Bandi, A. Dasari and V. Guttena, Acta Metall. Sin., 2016, 29, 17 CrossRef CAS.
- S. Sivakumar, A. Selvaraj, A. K. Ramasamy and V. Balasubramanian, Water, Air, Soil Pollut., 2013, 224, 1529 CrossRef.
- C. D. Lv, G. Chen, J. X. Sun, C. S. Yan, H. J. Dong and C. M. Li, RSC Adv., 2015, 5, 3767 RSC.
- C. M. Zhang, G. Chen, C. M. Li, J. X. Sun, C. D. Lv, S. Fan and W. N. Xing, ACS Sustainable Chem. Eng., 2016, 4, 5936 CrossRef CAS.
- D. J. Wang, Y. Z. Zhen, G. L. Xue, F. Fu, X. M. Liu and D. S. Li, J. Mater. Chem. C, 2013, 1, 4153 RSC.
- A. Etogo, R. Liu, J. B. Ren, L. W. Qi, C. C. Zheng, J. Q. Ning, Y. J. Zhong and Y. Hu, J. Mater. Chem. A, 2016, 4, 13242 CAS.
- F. D. Gao, D. W. Zeng, Q. W. Huang, S. Q. Tian and C. S. Xie, Phys. Chem. Chem. Phys., 2012, 14, 10572 RSC.
- P. T. Hsieh, Y. C. Chen, K. S. Kao and C. M. Wang, Appl. Phys. A: Mater. Sci. Process., 2008, 90, 317 CrossRef CAS.
- K. K. Banger, Y. Yamashita, K. Mori, R. L. Peterson, T. Leedham, J. Rickard and H. Sirringhaus, Nat. Mater., 2011, 10, 45 CrossRef CAS PubMed.
- C. Y. Liu, H. W. Huang, X. Du, T. R. Zhang, N. Tian, Y. X. Guo and Y. H. Zhang, J. Phys. Chem. C, 2015, 119, 17156 CAS.
- D. M. Chen, K. W. Wang, D. G. Xiang, R. L. Zong, W. Q. Yao and Y. F. Zhu, Appl. Catal., B, 2014, 147, 554 CrossRef CAS.
- H. W. Huang, X. Han, X. W. Li, S. C. Wang, P. K. Chu and Y. H. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 482 CAS.
- J. Cao, B. Y. Xu, H. L. Lin, B. D. Luo and S. F. Chen, Dalton Trans., 2012, 41, 11482 RSC.
- H. W. Huang, L. Y. Liu, Y. H. Zhang and N. Tian, RSC Adv., 2015, 5, 1161 RSC.
- W. S. D. Santos, L. D. Almeida, A. S. Afonso, M. Rodriguez, J. P. Mesquita, D. S. Monteiro, L. C. A. Oliveira, J. D. Fabris and M. C. Pereira, Appl. Catal., B, 2016, 182, 247 CrossRef.
- H. J. Huang, D. Z. Li, Q. Lin, W. J. Zhang, Y. Shao, Y. B. Chen, M. Sun and X. Z. Fu, Environ. Sci. Technol., 2009, 43, 4164 CrossRef CAS PubMed.
- Z. Y. Zhang, K. C. Liu, Z. Q. Feng, Y. N. Bao and B. Dong, Sci. Rep., 2016, 6, 19221 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nj03413j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |