Synthesis of Cu3(MoO4)2(OH)2 nanostructures by simple aqueous precipitation: understanding the fundamental chemistry and growth mechanism
Basudev
Swain†
 a,
Duk-Hee
Lee†
a,
Jae Ryang
Park
a,
Chan-Gi
Lee
a,
Kun-Jae
Lee
b,
Dong-Wan
Kim
*c and
Kyung-Soo
Park
*a
a,
Duk-Hee
Lee†
a,
Jae Ryang
Park
a,
Chan-Gi
Lee
a,
Kun-Jae
Lee
b,
Dong-Wan
Kim
*c and
Kyung-Soo
Park
*a
aAdvanced Materials & Processing Center, Institute for Advanced Engineering (IAE), Yongin, Korea. E-mail: kspark@iae.re.kr; Fax: +82 31 330 7113; Tel: +82 31 330 7422
bDepartment of Energy Engineering, Dankook University, Cheonan, Korea
cSchool of Civil, Environmental and Architectural Engineering, Korea University, Korea. E-mail: dwkim1@korea.ac.kr; Tel: +82 02 3290 4863
First published on 5th December 2016
Abstract
Lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers were synthesized through the simplest possible route by an aqueous chemical precipitation technique at room temperature without using any surfactants, template, expensive chemicals, complex instrumentation or tedious multistage synthesis process. Their morphology, structure, thermal properties, surface area, synthesis chemistry, and structural and growth mechanisms involved in the synthesis process were analyzed. Using XRD, FE-SEM, HR-TEM and FT-IR spectroscopy, their structure and morphology were analyzed. The thermal stability, surface area and porosity of the Cu3(MoO4)2(OH)2 nanoflowers were analyzed by TGA and BET. XRD analysis showed that the Cu3(MoO4)2(OH)2 nanoflowers have a pure monoclinic structure. The morphological analysis showed that the Cu3(MoO4)2(OH)2 nanoflowers are ∼10 μm in size, which are formed from self-assembly of thin nanosheets with a thickness of ∼20 nm. TGA indicated that the Cu3(MoO4)2(OH)2 nanoflowers are stable materials up to 328 °C and the isotherm from BET analysis indicated that the Cu3(MoO4)2(OH)2 nanoflowers are non-porous materials. The BET surface area of the synthesized Cu3(MoO4)2(OH)2 nanoflowers was found to be 21.357 m2 g−1. Moreover, the effects of the pH value and reaction time on the morphology of the Cu3(MoO4)2(OH)2 nanoflowers were studied and their optimization was performed. The results of the optimization study indicated that the reaction time and pH are two important parameters influencing the nucleation, growth, morphology, and synthesis mechanism. These flower-shaped Cu3(MoO4)2(OH)2 nanostructures are promising precursors for preparing molybdenum oxide materials which have various applications and can be synthesized in a very simple one-pot reaction system using commonly available chemicals without using a complex route.
1. Introduction
Lindgrenite, Cu3(MoO4)2(OH)2, is a naturally occurring uncommon material having hard ferrimagnet characteristics with a TC of 13 K and a coercive field of 4800 Oe at 2 K.1–3 Upon annealing, Cu3(MoO4)2(OH)2 can undergo a phase transformation to form tertiary copper molybdate (Cu3Mo2O9), which exhibits excellent magnetic and photocatalytic properties.4 The same material has outstanding performance in photocatalysis, photocurrent response and lithium storage batteries.5,6 Naturally occurring lindgrenite is contaminated with other impurities which make the material unfeasible to use for specific applications. Polyoxometalates including the tertiary copper molybdate (Cu3Mo2O9) nanomaterial have numerous advantages over bulk phase materials; hence, several authors have studied and reported polyoxometalate clusters, nanostructures, and nanomaterials in the literature.5,7–11 Various authors have reported the synthesis of nanostructured copper molybdate from 0D to 3D architecture, including nanoparticles, -rods, -plates, urchin-like structures, and hollow spheres, in the literature.12–15 In general, out-of-plane 3D nanostructures (nanoflowers) considerably enhance the intrinsic quality of the electric field, electromagnetic properties, optical properties, and light confinement by increasing the surface area.16–18 As 3D nanostructures enhance the photovoltaic (PV) solar cell efficiency, lithium storage properties and electromagnetic properties, hence, the synthesized lindgrenite Cu3(MoO4)2(OH)2 can be used for the same purpose.18–20Numerous authors have reported various nanostructured oxides, in general, metal oxides and, in particular, copper oxide and/or molybdenum oxide using diverse techniques.21–30 These metal oxide materials hold promise for several applications like photocatalysis, gas sensing and as cathode electrode materials for rechargeable lithium batteries. Unlike polyoxometalate nanomaterial and copper molybdate synthesis, reports regarding the synthesis of lindgrenite Cu3(MoO4)2(OH)2 are not much extensive but rather limited. The excellent energy application, photocatalytic and magnetic properties of Cu3(MoO4)2(OH)2 add motivation to synthesize, characterize and investigate its various applications.1–3,5,6 For the easier synthesis of copper molybdate or polyoxomolybdate nanomaterial, Cu3(MoO4)2(OH)2 can be an interesting intermediate compound. In the literature, Cu3(MoO4)2(OH)2 nanomaterial synthesis by various techniques has been reported. Table 1 summarizes Cu3(MoO4)2(OH)2 nanomaterial synthesis by different authors using various techniques which have been reported in the literature. Liu et al. have reported an electrochemistry-assisted laser ablation in liquid technique for Cu3(MoO4)2(OH)2 nanomaterial synthesis.5 The reported electrochemistry-assisted laser ablation technique required relatively complex instrumentation. Xia et al. have reported a hydrothermal reaction method for synthesis of Cu3(MoO4)2(OH)2 nanomaterial where ammonium molybdate tetrahydrate and copper acetates have been used as precursors and the reported technique is a multi-stage reaction process.6 Xu et al. have reported the room temperature synthesis of curved ammonium copper molybdate nanoflakes which can be further processed as Cu3(MoO4)2(OH)2 or Cu3Mo2O9. This method consists of a liquid–solid reaction between the Na2MoO4 solution and the copper substrate with the assistance of formamide. As Xu et al. have reported, the selective adsorption of formamide molecules plays a major role in determining the curved morphology and shaping the different crystallographic planes of ammonium copper molybdate.31 Jiang et al. have reported nano-sized tabular Cu3(MoO4)2(OH)2 synthesis by aqueous precipitation at 60 °C using Cu(NO3)2·6H2O, Na2MoO4·2H2O, and NaOH as precursors.32 Yan et al. have reported hollow and prickly sphere-like lindgrenite (Cu3(MoO4)2(OH)2) synthesis by both hydrothermal and solvothermal routes.33
| Synthesis method | Nanostructure | Chemical/process used | Advantages/disadvantages | References |
|---|---|---|---|---|
| Electrochemistry-assisted laser ablation in liquid (ECLAL) | Nanorods | MoO3 or MoO42− and copper anode | High temperature, high density, and high pressure | Liu et al.5 |
| Hydrothermal reaction followed by sintering | Monodisperse micropompons | Ammonium molybdate tetrahydrate (AMT) and metal acetates | Time intensive and energy intensive | Xia et al.6 |
| Liquid–solid reaction using a hierarchical architecture microreactor | Curved nanoflakes | Na2MoO4 solution, copper substrate and formamide | Nanogroove structures and organic solvent | Xu et al.31 |
| Aqueous precipitation | Nano-sized tabular structures | Cu(NO3)2·6H2O, Na2MoO4·2H2O and NaOH | Nitrate media | Jiang et al.32 |
| Hydrothermal and solvothermal | Hollow and prickly sphere-like nanostructures | CuCl2 and Na2MoO4 | Time intensive, energy intensive, and organic solvent | Yan et al.33 |
The above reported Cu3(MoO4)2(OH)2 syntheses have drawbacks, like a complex multistage synthesis process which adds disadvantages like purity and tediousness, involvement of organic chemicals which have environmental limitations, or complex instrumentation. In the reported Cu3(MoO4)2(OH)2 synthesis techniques, the hydrothermal route may be a simpler process,32 but our (here) reported process is even simpler. In this current synthesis, the Cu3(MoO4)2(OH)2 nanoflowers have been synthesized by a simple aqueous precipitation route at room temperature without using any surfactants or templates but rather from commonly available chemicals. The reported synthesis process is only a single-pot synthesis system where minimal chemical, virtually no energy or complex instrumentation has been employed. Rather controlling the reaction time and pH in the simplest way through a one-pot reactor system, the Cu3(MoO4)2(OH)2 spherical nanoflowers can easily be synthesized. Moreover, in the explored synthesis system, the reaction time and pH play an important role in the control of the morphology of the Cu3(MoO4)2(OH)2 nanoflowers. The novelties of the reported investigation are (i) simple one-pot synthesis without using any template or surfactant at room temperature, (ii) the possible chemistry and mechanism of Cu3(MoO4)2(OH)2 synthesis is discussed and (iii) the synthesis process has been optimized through pH variation and control of reaction time which add value for industrial production.
2. Experimental
Lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers were synthesized at room temperature by a simple aqueous chemical technique without using any templates and surfactants. Copper(II) acetate (Cu(CO2CH3)2·H2O) and sodium molybdate (Mo(VI)) (Na2MoO4) of 50 mM were prepared by dissolving in deionized water. The copper acetate solution was added dropwise into the sodium molybdate solution under constant stirring at room temperature. The reaction was maintained at room temperature for 16 min and the pH value of the as-prepared mixture demonstrated a weak acidic state (5.2). The obtained precipitates were washed with deionized water and acetone several times and then dried at 60 °C for 6 h in a vacuum oven.The crystal structures of the synthesized lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers were characterized by X-ray powder diffraction (XRD, Miniflex II, λCuKα = 1.5406 Å) with the range from 10° ≤ 2θ ≤ 60° and rate of 2° min−1. The morphology of the lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers was analyzed by field-emission scanning electron microscopy (FE-SEM, FEI NOVA, 10 kV) and high-resolution transmission electron microscopy (HR-TEM, JEM-2100F, 200 kV). The same structures were also analyzed using a Fourier transform infrared spectrometer (FT-IR, model Nicolet 5700) in the range of 400–4000 cm−1. Characterization techniques like nitrogen adsorption–desorption for the BET surface area, pore size distribution, and thermal stability studies have been used to elucidate the physicochemical properties of the Cu3(MoO4)2(OH)2 nanoflowers. The thermal stability, surface area and porosity of the synthesized Cu3(MoO4)2(OH)2 nanoflowers were analyzed by TGA (TGA: DTG-60A, Shimadzu, Japan) and BET analysis (BELSORP-mini II, BEL, Japan), respectively.
3. Results and discussion
3.1. Characterization of Cu3(MoO4)2(OH)2 nanoflowers
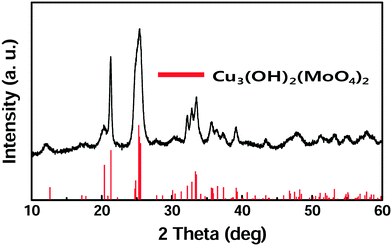
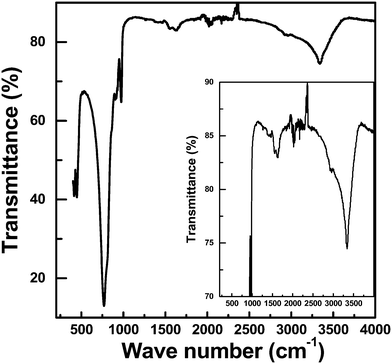
Lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers were synthesized from the simple mixing of aqueous solutions containing copper(II) acetate (Cu(CO2CH3)2·H2O) and sodium molybdate (Na2MoO4). When the Cu(CO2CH3)2·H2O aqueous solution was added to the Na2MoO4 solution under stirring, a sky-blue colored colloid was formed initially, and then a light-green colored precipitate was formed. The obtained precipitates were analyzed by various techniques as explained above. As seen in Fig. 1, the XRD pattern of the obtained sample well corresponded to the monoclinic lindgrenite (JCPDS no. 86-2311) with no diffraction peaks of amorphous molybdenum, copper compounds, and another phase. As shown in the figure, almost all peaks were overlapping and broadened, which was possibly attributed to the low crystallinity and/or small crystallite size of Cu3(MoO4)2(OH)2. All the Bragg reflections have been indexed using the pattern matching with PDF card no. 0100862311, showing that the unit cell parameters a = 5.613 Å, b = 14.030 Å, c = 5.405 Å, α = 90.00°, β = 98.38°, and γ = 90.00° are in very good agreement. The pattern matching with the PDF card indicated that the sample is a monophasic material having a monoclinic space group of P21/n(14). Fig. 2 shows the FT-IR spectra of Cu3(MoO4)2(OH)2 and the inset figure shows a higher resolution of the unclear part of the FT-IR spectra. Table 2 lists the fundamental vibrational frequencies of various possible bonds and observed frequencies of different possible bonds in the synthesized Cu3(MoO4)2(OH)2. For Cu3(MoO4)2(OH)2, the transmittance band at 450 cm−1 is assigned to the Cu–OH vibration band of Cu3(MoO4)2(OH)2, and four bands (located between 810 and 972 cm−1) are ascribed to MoO42− (the 972 cm−1 band to ν1MoO42− and the 916, 865, and 810 cm−1 bands to ν3MoO42−) consistent with the reported values.24,25 Moreover, the weak band at 1632 cm−1 and the band at 3340 cm−1 may be due to the infrared vibration of OH and H–OH (hydrogen bonding), respectively.20| Compound | Fundamental vibrational frequencies, cm−1 | Observed vibrational frequencies, cm−1 |
|---|---|---|
| O–H | 3340 | |
| H–O–H | 1632 | |
| MoO4− | 958 | 972 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
| MoO4− | 897 | 916 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
| MoO4− | 317 | MoO4− |
| MoO4− | 837 | 865 (Mo–O) |
| MoO4− | 317 | MoO4− |
| MoO4− | 837 | 865 (Mo–O) |
| Cu–OH | 455, 440 | 450 |
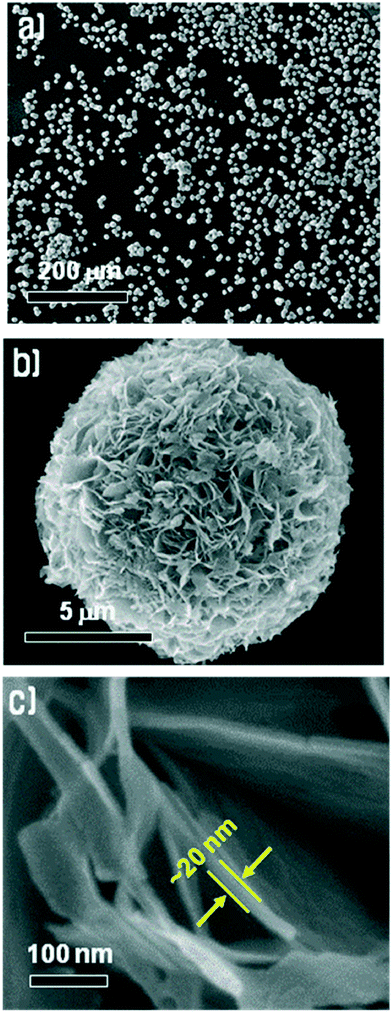
Fig. 3 shows an FE-SEM image of the Cu3(MoO4)2(OH)2 synthesized under the conditions mentioned above at various resolutions. Fig. 3a shows that the synthesized Cu3(MoO4)2(OH)2 nanostructures are spherically shaped with a size of ∼10 μm and are well dispersed without severe agglomeration. In fact, agglomeration rarely occurred. As shown in Fig. 3b, at an intermediate resolution, the Cu3(MoO4)2(OH)2 spheres are nanoflowers and these nanoflowers are composed entirely of many thin nanosheets of Cu3(MoO4)2(OH)2. Fig. 3c clearly shows that the spherical Cu3(MoO4)2(OH)2 nanoflowers are composed of thin nanosheets of Cu3(MoO4)2(OH)2 with a thickness of ∼20 nm. It is known that the crystal structure of Cu3(MoO4)2(OH)2 is similar to that of malachite (Cu2(CO3)(OH)2), which has a layer structure, therefore it can readily form a two-dimensional plate shape without using any templates.19
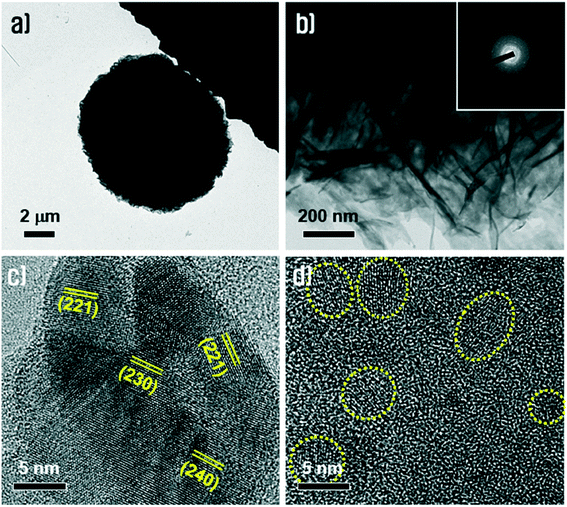
The morphology and crystallinity of the Cu3(MoO4)2(OH)2 nanoflowers were further investigated using TEM and HR-TEM, as shown in Fig. 4. Fig. 4a shows the TEM of the Cu3(MoO4)2(OH)2 nanoflowers and Fig. 4b shows the TEM of the nanosheets. The combination of Fig. 4a and b reasonably justifies that an individual Cu3(MoO4)2(OH)2 nanoflower with a diameter of ∼10 μm is composed of very thin, paper-like, rumpled nanosheets with a thickness of ∼20 nm. The nanosheets have a polycrystalline nature as analyzed in a SAED pattern corresponding to a region in Fig. 4b (inset of Fig. 4b). Such a nature could also be evidenced and identified in the HR-TEM images (Fig. 4c and d). The HR-TEM of Cu3(MoO4)2(OH)2 (Fig. 4c) shows that the nanosheets have typical crystalline morphologies which are composed of polycrystalline grains having random crystallographic planes such as (221), (230), and (240). Fig. 4d shows nanodomains with sizes of ∼5 nm embedded in an amorphous matrix. These HR-TEM results can be evidence for the broad peaks obtained in the XRD pattern.
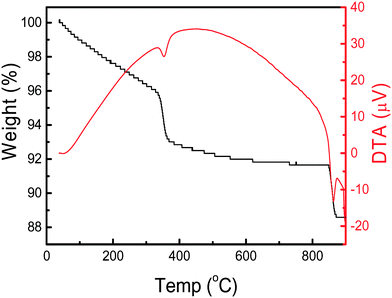
The thermal characteristics of the synthesized Cu3(MoO4)2(OH)2 nanoflowers are depicted in Fig. 5. The TGA curve in Fig. 5 shows that two distinct transitions (weight loss stages) appear at 383 and 902 °C, which correspond to the DTA troughs in the DTA curve. The first transition in the TGA curve shows a weight loss of 3.24% in the temperature range of 328–383 °C corresponding to a theoretical weight loss of (3.30%) a water molecule from Cu3(OH)2(MoO4)2. The TGA curve provides two important pieces of information, i.e., (i) the Cu3(MoO4)2(OH)2 nanoflowers are stable materials up to 328 °C, and (ii) by thermal annealing, the Cu3(OH)2(MoO4)2 can be converted to Cu3Mo2O9. Cu3Mo2O9 is an excellent photocatalyst and magnetic material which can be used in several applications.4–6 In the DTA curve, an endothermic valley in the temperature range of 328–383 °C indicates the loss of a water molecule from Cu3(OH)2(MoO4)2 and formation of Cu3Mo2O9.
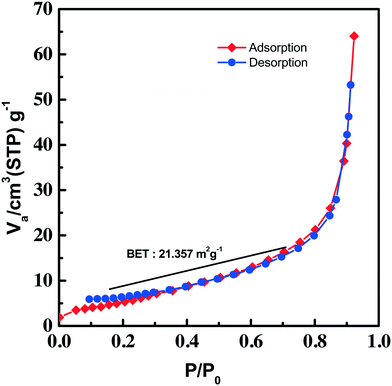
An adsorption–desorption (nitrogen) isotherm is constructed from the BET surface area measurement and depicted in Fig. 6. The isotherm given in the figure can be categorized as type III, which has a weak adsorbate–adsorbent interaction. The absence of any adsorption–desorption hysteresis in the isotherm rules out the mesoporous behavior of the synthesized Cu3(MoO4)2(OH)2. The isotherm indicated multilayer gas adsorption in the non-porous material. The BET surface area of the synthesized Cu3(MoO4)2(OH)2 nanoflowers was found to be 21.357 m2 g−1.
3.2. Understanding the aqueous chemistry
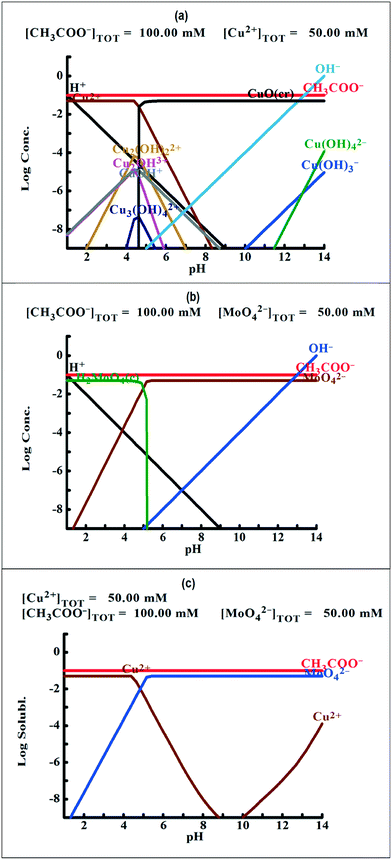
To understand the color changes during synthesis, the possible chemistry involved in the Cu3(MoO4)2(OH)2 nanoflower synthesis and thermodynamically possible species were analyzed through speciation diagrams. The possible speciation behavior of Cu(CO2CH3)2·H2O and Na2MoO4 in aqueous solution as a function of pH at 25 °C under 1 atm pressure was analyzed using Medusa–Hydra chemical equilibrium software, developed by the School of Chemical Science and Engineering, KTH, Stockholm, Sweden. Fig. 7a shows all the possible species of Cu in the acetate medium and Fig. 7b shows all the molybdate species in the aqueous solution as a function of pH. Fig. 7a shows that Cu(CO2CH3)2·H2O dissociates into Cu2+ and CH3COO− followed by hydrolysis of Cu2+. As a function of pH, the hydrolysis behavior of Cu2+ varies. At acidic pH, Cu2+ hydrolyses into Cu2(OH)22+, Cu2(OH)3+, Cu(OH)+, and Cu3(OH)42+. The same figure indicates that the concentration of all Cu2(OH)22+, Cu2(OH)3+, Cu(OH)+, and Cu3(OH)42+ species peaks near pH 4.5 to 4.0. Similarly, Fig. 7b shows that the molybdate mostly exists as MoO42−, but at low pH (in the pH range of 0–5) as H2MoO4, and the same species exists as MoO42− at higher pH (beyond pH 5). Considering the experimental pH (5.2) and speciation diagram, the precursor Cu2+ either exists as Cu2(OH)3+ or Cu(OH)+ or all together, and the molybdenum exists as MoO42− at pH 5.2. Fig. 7c represents the solubility behavior of cupric species and molybdate species as a function of pH at standard temperature and pressure using 50 mM copper(II) acetate (Cu(CO2CH3)2) and 50 mM sodium molybdate (Na2MoO4). From XRD, the synthesized nanoflower material was identified to be Cu3(MoO4)2(OH)2 nanoflowers having a monoclinic structure. Based on the above information, the possible aqueous precipitation synthesis of copper lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers can be explained step by step as given below.| Cu(CO2CH3)2 ⇌ Cu2+ + 2CO2CH3− | (1) |
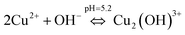 | (2) |
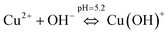 | (3) |
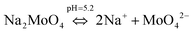 | (4) |
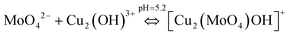 | (5) |
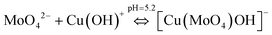 | (6) |
Combining eqn (5) and (6), the chemical reaction for synthesis of lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers can be represented as eqn (7).
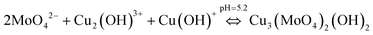 | (7) |
| 2CO2CH3− + 2Na+ ⇌ 2CO2CH3Na | (8) |
The overall chemical reaction can be explained by eqn (9).
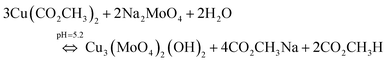 | (9) |
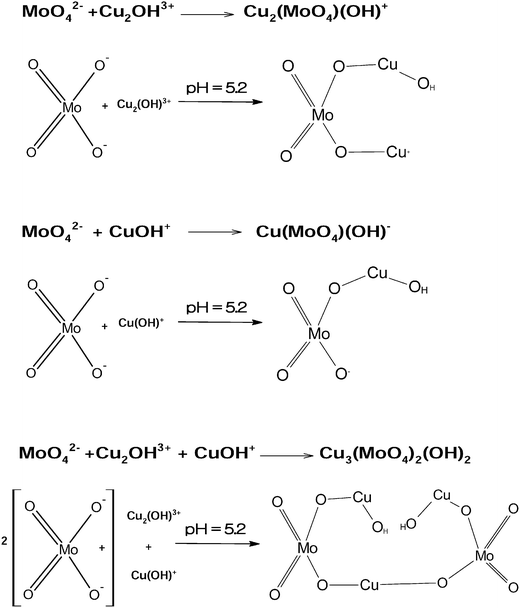
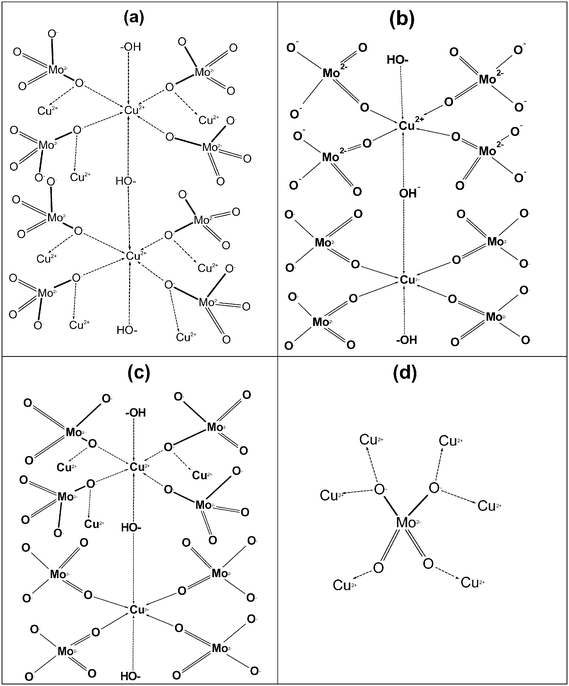
As explained in eqn (5)–(9), at acidic pH, sodium acetate and acetic acid (from eqn (9)) together act as a buffer as well as a stabilizing reagent in the aqueous synthesis of lindgrenite (Cu3(MoO4)2(OH)2) nanoflower synthesis. Based on XRD data and IR vibrational spectra, a possible structural mechanism involved in the synthesis has been elucidated and is presented in Fig. 8. The ionic reaction mechanism explained in Fig. 8 holds good only from the stoichiometry perspective, but from the monoclinic crystal structure perspective, Fig. 8 can hardly explain the possible synthesis mechanism.
3.3. Optimization and growth mechanism
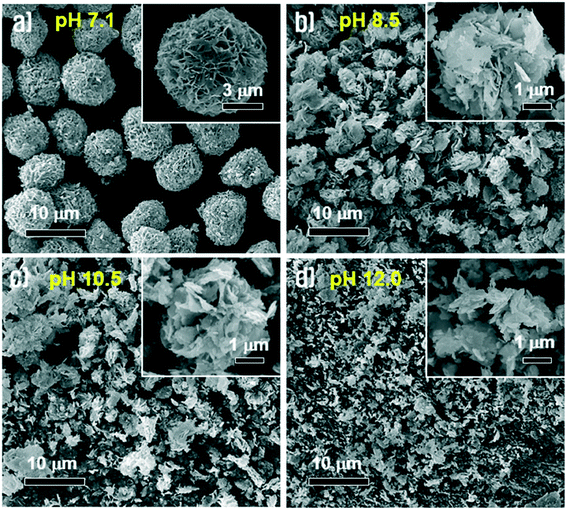
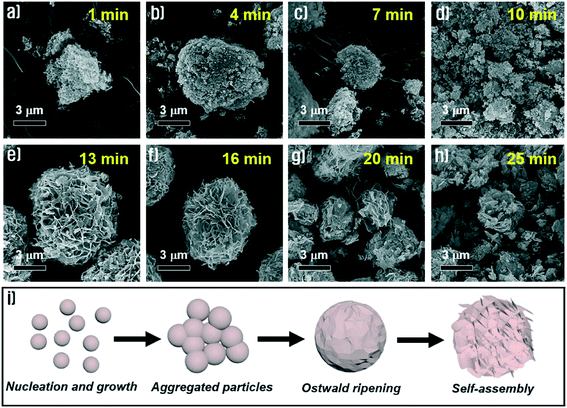
To identify the effect of pH on morphological changes, we carried out a synthetic process using sodium molybdate solution at different pH values, which were adjusted by addition of HCl or NaOH aqueous solutions before adding Cu(CO2CH3)2 solution. In the case of strong acidic conditions below pH 4.5, the Cu3(MoO4)2(OH)2 precipitates were not obtained because the nucleation process hardly initiated at this pH. On the other hand, the Cu3(MoO4)2(OH)2 precipitates were very well synthesized under near neutral and alkaline conditions. Fig. 9(a–d) show the morphologies of the synthesized Cu3(MoO4)2(OH)2 at pH = 7.1, pH = 8.5, pH = 10.5 and pH = 12.0, respectively. The figures indicated by increasing pH values from 7.1 to 12.0 show that the morphologies of the prepared Cu3(MoO4)2(OH)2 gradually transformed from complete nanoflowers to decomposed particles. As the pH of the aqueous solution increases, the Cu3(MoO4)2(OH)2 nanoflowers gradually lose their uniformity because the nanosheets are more randomly diffused among one another. At higher pH, as the buffer activity of sodium acetate is nullified, the aqueous chemistry of the Cu2+ solution gradually changes, thus the synthesis mechanism is the primary reason for the formation of non-homogeneous nanoflowers. Because of incomplete Ostwald ripening and self-assembly induced by intense nucleation at higher pH (containing more OH− ions), the Cu3(MoO4)2(OH)2 nanoflowers gradually lose their uniformity.To understand and investigate the possible growth mechanism, Cu3(MoO4)2(OH)2 nanoflowers were synthesized through a series of time-dependent synthesis processes, keeping all other conditions constant. The synthesized Cu3(MoO4)2(OH)2 nanoflowers were analyzed by FE-SEM. Fig. 10 compares the morphological evolution process of the synthesized Cu3(MoO4)2(OH)2 nanoflowers with reaction time at room temperature. As the reaction time was increased from 1 to 10 min, remarkable morphological changes of the obtained products were not observed and particles were usually agglomerated (Fig. 10a–d). In other words, the Cu3(MoO4)2(OH)2 samples synthesized at these reaction times are mainly micro-agglomerates containing a very small number of nanosheets that were formed by Ostwald ripening of nanoparticles. However, by increasing the reaction time to 13 min, the morphology of the Cu3(MoO4)2(OH)2 was changed to micron-sized nanoflowers (Fig. 10e). In this reaction time, well-defined nanoflowers were assembled from Cu3(MoO4)2(OH)2 nanosheets and no agglomerated nanoparticles could be observed. Although the nanoflowers maintained their unique morphology until the reaction time of 16 min (Fig. 10f), with increasing reaction time up to 25 min, the nanoflowers decomposed gradually into completely collapsed nanostructures (Fig. 10g and h), which is possibly ascribed to etching effects on amorphous regions by weak acidic conditions (pH 5.2) of the reaction solution. Based on the experimental results, the schematic illustration for the formation of such a nanoflower morphology (Fig. 10i) and the possible growth mechanism originated from the following three reasons. (i) When the Cu(CO2CH3)·H2O solution was dropped into the Na2MoO4·2H2O solution at the point of supersaturation of Cu2(OH)3+ and Cu(OH)+, nucleation started and led to rapid nucleation, followed by combination of Cu2(OH)3+ and Cu(OH)+ nuclei with MoO42− ions in the solution to form Cu3(MoO4)2(OH)2 nanoparticles. (ii) The small nanoparticles of Cu3(MoO4)2(OH)2 aggregated with each other and Ostwald ripening of the particles occurred leading to the formation of the nanosheets. (iii) The nanosheets self-assembled into a spherical shape by themselves to minimize the thermodynamic energy and the flower-like structures began to appear. Finally, well-defined Cu3(MoO4)2(OH)2 nanoflowers were finally produced by gradual assembly of the nanosheets.
3.4. Review of structure and analysis of synthesis mechanism
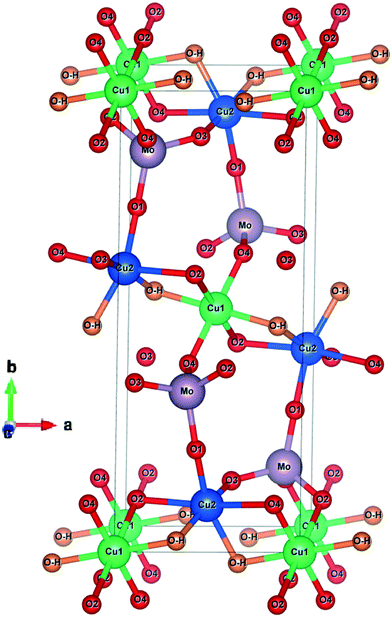
From XRD and FT-IR, reasonable similarities of our synthesized Cu3(MoO4)2(OH)2 and reported Cu3(MoO4)2(OH)2 (Vilminot et al.1) were observed. Hence, based on Vilminot et al., structural aspects of the synthesized Cu3(MoO4)2(OH)2 have been reviewed. Vilminot et al. have investigated the detailed structure and magnetic properties originating from the monoclinic structure of synthetic Cu3(MoO4)2(OH)2 in their study which is an important investigation reviewed here. Fig. 11 reflects (which has been reproduced from Vilminot et al.1) the detailed crystal structure and possible bonding of Cu3(MoO4)2(OH)2 based on neutron powder diffraction and IR spectral data analyses. The Cu3(MoO4)2(OH)2 crystal structure basically consists of two distorted octahedral Cu and one tetrahedral Mo having differently bonded O as ligands. Both Cu and Mo arranged in the crystal structure through different bonding modes and results in the monoclinic crystal of Cu3(MoO4)2(OH)2. As explained by Vilminot et al., in lindgrenite, the Cu3(OH)24+ ribbons (Fig. 11a) arranged in a brucite structure where Cu and O of MoO4 share the edges. The octahedral Cu3(OH)24+ ribbon runs parallel along the c-axis as shown in Fig. 11b. In the Cu3(OH)2 ribbons, two types of distorted octahedral copper atoms coordinated with O from MoO42−. Each ribbon alternately contains one Cu(1) octahedron and two Cu(2) octahedra, all having shared edges (Fig. 11). The octahedral Cu atoms are strongly distorted with a 4 + 2 environment. Each unit of octahedral Cu and ribbons is connected into 3D chains by a herringbone arrangement. The O in MoO42− plays a vital role in connecting ribbons in parallel and it is connected to a 3D network with octahedral Cu and makes up the monoclinic Cu3(MoO4)2(OH)2. In MoO42−, the oxygen atoms O(1) (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and O(3) (Mo
O) and O(3) (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are bonded to single Cu atoms, while O(2) (Mo–O−) and O(4) (Mo–O−) form a bridge bond between two Cu atoms each in two adjacent chains. Oxygen atoms are part of the monoclinic skeleton of the Cu3(MoO4)2(OH)2 in the ribbons. As explained in Fig. 11c, the MoO42− has a distorted tetrahedral structure, which shares its oxygen corners with six copper atoms.
O) are bonded to single Cu atoms, while O(2) (Mo–O−) and O(4) (Mo–O−) form a bridge bond between two Cu atoms each in two adjacent chains. Oxygen atoms are part of the monoclinic skeleton of the Cu3(MoO4)2(OH)2 in the ribbons. As explained in Fig. 11c, the MoO42− has a distorted tetrahedral structure, which shares its oxygen corners with six copper atoms.
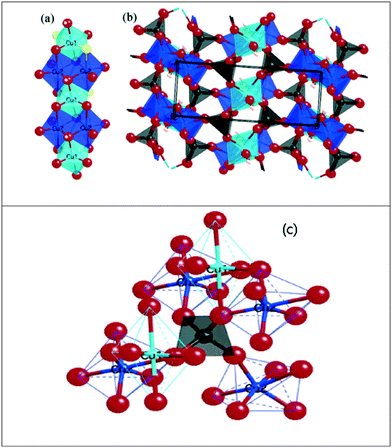 | ||
| Fig. 11 (a) Single ribbon showing the edge-sharing octahedral copper using coppers from two different environments. (b) The structure along the ribbons (c axis) showing the herringbone arrangement and the connection of the ribbons to the 3D network of MoO4−. (c) The coordination of the 3D network of MoO4− to its three neighboring ribbons of copper hydroxide. Reproduced from Vilminot et al.1 | ||
The possible chemical mechanism explained in Fig. 8 and eqn (5)–(9) partially explains the structural arrangement observed in Fig. 11. Considering the limitation of the ionic mechanism explained in Fig. 8, the Cu3(MoO4)2(OH)2 synthesis mechanism can be best explained using the coordination chemistry of Cu2+ and MoO42−. The (4 + 2) distorted octahedral coordination chemistry of MoO42− + Cu2(OH)3+ can be explained by eqn (10) and the possible mechanism can be explained as demonstrated in Fig. 12(a).
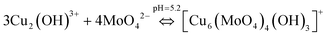 | (10) |
Similarly, the (4 + 2) distorted octahedral coordination chemistry of MoO42− + Cu(OH)+ can be explained by eqn (11) and the possible mechanism can be explained as demonstrated in Fig. 12(b).
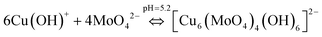 | (11) |
Finally, as explained in Fig. 11a, the ribbons are framed by two units of Cu from an environment and one unit of Cu from another environment. Hence, based on the structure explained by Vilminot et al., the total coordination chemistry can be explained by eqn (12), given below, and the structure can be explained using Fig. 12c.
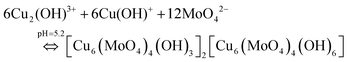 | (12) |
The coordination chemistry of MoO42− can be better explained as shown in Fig. 12d. Fig. 12 and the mechanism explained in eqn (12) clearly indicate that the Cu2+ in Cu2(OH)3+ and Cu(OH)+ exists in two different environments which was also clearly explained by Vilminot et al. Similarly, the OH− in Cu2(OH)3+ and Cu(OH)+, and O2− in MoO42− exist in two different environments each, which has not been discussed critically in Fig. 11 (Vilminot et al.1). Based on all the information, the Cu3(MoO4)2(OH)2 crystal structure has been redrawn and is depicted in Fig. 13. Fig. 13 clearly identifies each element and its environment.
4. Conclusions
Cu3(MoO4)2(OH)2 nanoflowers have been synthesized by a simple aqueous precipitation route at room temperature without using surfactants or templates from commonly available chemicals. By controlling the reaction time and pH in the simplest way through a one-pot reaction system, spherical nanoflowers could be synthesized. The XRD pattern, SEM and TEM images of the synthesized nanopowder showed that it has a monoclinic lindgrenite structure. Reasonably, from the TGA curve, the following conclusions can be inferred, i.e., (i) the Cu3(MoO4)2(OH)2 nanoflowers are stable materials up to 328 °C, and (ii) by thermal annealing (383 °C), the Cu3(OH)2(MoO4)2 can be converted to Cu3Mo2O9, which has excellent magnetic and photocatalytic properties. The BET surface area of the Cu3(MoO4)2(OH)2 nanoflowers is 21.357 m2 g−1. The flower-like nano-Cu3(MoO4)2(OH)2 resulted from the aggregation of nanosheets. Several 20 nm sheets through aggregation formed spherically shaped nanoflowers of ∼10 μm size. By our reported aqueous precipitation method, high purity Cu3(MoO4)2(OH)2 nanoflowers having a monoclinic lindgrenite structure can be easily synthesized in the simplest possible way. The reported aqueous chemical precipitation method to synthesize nanostructured Cu3(MoO4)2(OH)2 can be a promising route because of its short reaction time, high purity, non-tedious process and use of non-hazardous chemicals, which eliminate complex multistep synthesis systems or complex instrumentation. The synthesis process also adds scope for the synthesis of the Cu3Mo2O9 nanomaterial.Acknowledgements
This study was supported by the Korea Ministry of the Environment under ‘Global-top program’ (2016002250005).References
- S. Vilminot, G. André, M. Richard-Plouet, F. Bourée-Vigneron and M. Kurmoo, Inorg. Chem., 2006, 45, 10938–10946 CrossRef CAS PubMed
.
- Lindgrenite, http://www.mindat.org/min-2405.html, (accessed 1/8/2016, 2016)
.
- M. P. Shores, B. M. Bartlett and D. G. Nocera, J. Am. Chem. Soc., 2005, 127, 17986–17987 CrossRef CAS PubMed
.
- S. Vilminot, G. Andre and M. Kurmoo, ChemInform, 2009, 40 DOI:10.1002/chin.200923010
.
- P. Liu, Y. Liang, X. Lin, C. Wang and G. Yang, ACS Nano, 2011, 5, 4748–4755 CrossRef CAS PubMed
.
- J. Xia, L. X. Song, W. Liu, Y. Teng, Q. S. Wang, L. Zhao and M. M. Ruan, RSC Adv., 2015, 5, 12015–12024 RSC
.
- D.-L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105–121 RSC
.
- T. Zhang, J. Brown, R. J. Oakley and C. F. J. Faul, Curr. Opin. Colloid Interface Sci., 2009, 14, 62–70 CrossRef CAS
.
- P. Kögerler and L. Cronin, Angew. Chem., Int. Ed., 2005, 44, 844–846 CrossRef
.
- H.-Y. Zang, A. R. de la Oliva, H. N. Miras, D.-L. Long, R. T. McBurney and L. Cronin, Nat. Commun., 2014, 5 DOI:10.1038/ncomms4715
.
- D. Fan, J. Hao and Q. Wei, J. Inorg. Organomet. Polym. Mater., 2012, 22, 301–306 CrossRef CAS
.
- M. R. Montney, J. G. Thomas, R. M. Supkowski, R. J. Trovitch, J. Zubieta and R. L. LaDuca, Inorg. Chem. Commun., 2009, 12, 534–539 CrossRef CAS
.
- Z. Shahri, M. Salavati-Niasari, N. Mir and G. Kianpour, J. Cryst. Growth, 2014, 386, 80–87 CrossRef CAS
.
- M. Rahimi-Nasrabadi, S. M. Pourmortazavi and M. Khalilian-Shalamzari, J. Mol. Struct., 2015, 1083, 229–235 CrossRef CAS
.
- A. Moini, R. Peascoe, P. R. Rudolf and A. Clearfield, Inorg. Chem., 1986, 25, 3782–3785 CrossRef CAS
.
- M. Malerba, A. Alabastri, E. Miele, P. Zilio, M. Patrini, D. Bajoni, G. C. Messina, M. Dipalo, A. Toma, R. Proietti Zaccaria and F. De Angelis, Sci. Rep., 2015, 5, 16436 CrossRef CAS PubMed
.
- G. Lui, J.-Y. Liao, A. Duan, Z. Zhang, M. Fowler and A. Yu, J. Mater. Chem. A, 2013, 1, 12255–12262 CAS
.
- J. Liu, M.-S. Cao, Q. Luo, H.-L. Shi, W.-Z. Wang and J. Yuan, ACS Appl. Mater. Interfaces, 2016, 8, 22615–22622 CAS
.
- Z. Fan, H. Razavi, J.-W. Do, A. Moriwaki, O. Ergen, Y.-L. Chueh, P. W. Leu, J. C. Ho, T. Takahashi, L. A. Reichertz, S. Neale, K. Yu, M. Wu, J. W. Ager and A. Javey, Nat. Mater., 2009, 8, 648–653 CrossRef CAS PubMed
.
- B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146–17148 CrossRef CAS PubMed
.
- X. Zou, H. Fan, Y. Tian and S. Yan, CrystEngComm, 2014, 16, 1149–1156 RSC
.
- X. Zou, H. Fan, Y. Tian, M. Zhang and X. Yan, Dalton Trans., 2015, 44, 7811–7821 RSC
.
- X. Zou, H. Fan, Y. Tian, M. Zhang and X. Yan, RSC Adv., 2015, 5, 23401–23409 RSC
.
- B. Gao, H. Fan and X. Zhang, J. Phys. Chem. Solids, 2012, 73, 423–429 CrossRef CAS
.
- W. Yan, H. Fan, Y. Zhai, C. Yang, P. Ren and L. Huang, Sens. Actuators, B, 2011, 160, 1372–1379 CrossRef CAS
.
- S. G. Hosseini and R. Abazari, RSC Adv., 2015, 5, 96777–96784 RSC
.
- Y.-M. Juan, S.-J. Chang, H.-T. Hsueh, S.-H. Wang, T.-C. Cheng, S.-W. Huang and C.-L. Hsu, RSC Adv., 2015, 5, 54220–54224 RSC
.
- X. Zhang, Y. Yang, W. Que and Y. Du, RSC Adv., 2016, 6, 81607–81613 RSC
.
- D. Li, J. Xue, J. Ma and J. Tang, New J. Chem., 2016, 40, 3330–3335 RSC
.
- J. Jiang, J. Liu, S. Peng, D. Qian, D. Luo, Q. Wang, Z. Tian and Y. Liu, J. Mater. Chem. A, 2013, 1, 2588–2594 CAS
.
- J. Xu, D. Xue and Y. Zhu, J. Phys. Chem. B, 2006, 110, 17400–17405 CrossRef CAS PubMed
.
- W.-J. Jiang, J. Fang, Z.-Y. Fan, X.-J. Yang, Q.-T. Lu and Y.-B. Hou, J. Inorg. Mater., 2011, 26, 438–442 CrossRef CAS
.
- C. Yan, L. Zou, D. Xue, J. Xu and M. Liu, J. Mater. Sci., 2008, 43, 2263–2269 CrossRef CAS
.
Footnote |
| † Basudev Swain and D. H. Lee are both equally contributing authors. |
| This journal is © The Royal Society of Chemistry 2017 |