Synthesis of very thin Ag nanowires with fewer particles by suppressing secondary seeding†
Dan
Jia
ab,
Yan
Zhao
c,
Wei
Wei
c,
Chao
Chen
ab,
Guowei
Lei
c,
Mengjuan
Wan
ab,
Jingqi
Tao
ab,
Shuxin
Li
a,
Shulin
Ji
*a and
Changhui
Ye
*a
aKey Laboratory of Materials Physics and Anhui Key Laboratory of Nanomaterials and Technology, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, China. E-mail: slji@issp.ac.cn; chye@issp.ac.cn
bUniversity of Science and Technology of China, Hefei 230026, China
cShaanxi Coal and Chemical Technology Institute Co. Ltd., Xian 710065, Shaanxi, China
First published on 21st November 2016
Abstract
The synthesis of very thin Ag nanowires in the presence of Br− ions is typically accompanied by the generation of a huge amount of Ag nanoparticles. Herein, we report a method to suppress the homogeneous nucleation of Ag seeds in the growth process of Ag nanowires, hence dramatically enhancing the purity of the synthesized very thin Ag nanowires. These Ag nanowires having a diameter approaching 20 nm and an aspect ratio larger than 1500, as well as an ultra-low particle impurity level of 5.5% are directly used after simple water washing to form transparent conductive films with much improved optical and electrical properties, which have great potential for high-end applications including touch panels.
Introduction
Silver nanowires (AgNWs) have drawn tremendous attention as the most promising candidate for building flexible transparent conductive films (TCFs).1–6 Very thin silver nanowires (VT-AgNWs) are extremely important for fabricating high quality TCFs with high visible light transmittance, low sheet resistance, and low haze factor.7–10 Previously, several research groups have reported the synthesis of thin AgNWs with a diameter smaller than 40 nm.11–13 Although at this size regime one could fabricate TCFs with excellent transparency and conductance, the haze factor is still larger than what is required for high-end touch panel applications. Recently, synthesis of VT-AgNWs with a diameter of ∼20 nm under the coexistence of Cl− and Br− ions has been reported.14–17 Unfortunately, the reported growth process either involved high pressure or was accompanied by the generation of huge amounts of Ag nanoparticles (NPs). The post-growth purification procedure is rather time-consuming, environmentally harmful, and less cost-effective. Therefore, it is extremely important to elucidate the growth aspects of VT-AgNWs, suppress the homogeneous nucleation (secondary seeding) of Ag NPs, and improve the purity of VT-AgNWs.VT-AgNWs were typically synthesized by a polyol reduction method under the coexistence of Cl− and Br− ions.16,17 It was believed that Cl− ions favor the formation of pentagonally twinned Ag seeds, while Br− ions inhibit overgrowth of the seeds.16–20 Cl− ions play their role in electrostatically stabilizing Ag seeds against aggregation through being stochastic on low-index facets of Ag,21 while Br− ions have their key function in further reducing the seed size by increasing the number of nucleation events due to their excessive nucleation ability.16,17 Cl− and Br− could combine with Ag+ to form AgCl and AgBr, hence competing for Ag+ with ethylene glycol.20,22 Generally, large particles up to several hundred nanometers in diameter composed of AgCl, AgBr, or even a mixture of them were produced at first. These NPs acted as heterogeneous nucleation sites for Ag seeds in a later seeding period.23–25 The seeds that survived as multiply twinned particles (MTPs) subsequently evolved into AgNWs with the addition of Ag atoms via surface catalyzed Ag+ reduction.20,25 Some researchers believed that the silver mainly came from the AgNO3 precursor;16,17,24,25 however, Zhan et al. found that Ag from AgCl NPs could also make a great contribution.23 At least one consensus could be reached, that is, the AgCl as well as AgBr NPs formed previously would dissolve or be consumed accompanying the growth of AgNWs. Besides, it has been suggested that the geometric parameters, such as the diameter, length, and aspect ratio of AgNWs, did not depend on the size of silver halide NPs where AgNWs evolved; instead, the size of AgNWs increased with time prior to reaching a plateau.25 However, the co-occurrence of huge amounts of Ag NPs accompanying VT-AgNWs is troublesome.16,17 So far, there has been rather limited knowledge on how these NPs formed and how to suppress their formation; synthesis of VT-AgNWs with fewer NPs is urgently needed.
Herein, we report the synthesis of VT-AgNWs with fewer particles by suppressing secondary seeding. Homogeneous nucleation requires a certain driving force, that is, a suitable temperature and precursor concentration. When the temperature decreases, both the homogeneous nucleation and the surface catalyzed reduction (the growth of AgNWs) rates decrease, with the former being affected to a larger extent.19 If the synthesis could reach a status in which the AgCl and AgBr precursor NPs could be mostly removed while secondary seeding does not take place extensively, reducing the reaction temperature to that point should lead to the formation of fewer NPs in the product. As will be shown in the following section, we can dramatically reduce the population of Ag NPs and obtain VT-AgNWs with a diameter of ∼21 nm and a length of ∼34 μm.
Experimental
All reagents were used as-received without further purification. AgNO3 (≥99.8%, Shanghai Qiangshun Chemical Reagent Co., Ltd.) and ethylene glycol (EG, Sinopharm Chemical Reagent Co., Ltd.) were used as the source material and the reductant. Poly(vinylpyrrolidone) (PVP, average molecular weight (MW) of 360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, PVP-360
000, PVP-360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich) was used as the capping agent. NaCl (≥99.5%, Sinopharm Chemical Reagent Co., Ltd.) and NaBr (≥99%, Sinopharm Chemical Reagent Co., Ltd.) were used as nucleating agents. Hydroxypropyl methylcellulose (HPMC, Sigma-Aldrich), Sago-dispersant and Sago-flatting agent (Sago Co., Ltd.) were used for AgNW ink formation before film coating.
000, Sigma-Aldrich) was used as the capping agent. NaCl (≥99.5%, Sinopharm Chemical Reagent Co., Ltd.) and NaBr (≥99%, Sinopharm Chemical Reagent Co., Ltd.) were used as nucleating agents. Hydroxypropyl methylcellulose (HPMC, Sigma-Aldrich), Sago-dispersant and Sago-flatting agent (Sago Co., Ltd.) were used for AgNW ink formation before film coating.
Silver nanowires were synthesized by a polyol process. EG (38.5 mL), NaBr solution (0.0114 g in 0.5 mL of EG), NaCl solution (0.0123 g in 1 mL of EG), PVP-360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 solution (0.2800 g in 5 mL of EG) and fresh AgNO3 solution (0.2255 g in 5 mL of EG) were successively added into a 100 mL flask placed in an oil bath at room temperature. The solution was moderately stirred for 30 min, and then slowly heated to 180 °C in 10–20 min. Nitrogen gas (150 mL min−1) was bubbled through the reaction during heating. Once the temperature reached 180 °C, the solution was naturally cooled to a certain temperature, such as 150–170 °C without stirring. After reaction for a period, the solution was cooled to room temperature.
000 solution (0.2800 g in 5 mL of EG) and fresh AgNO3 solution (0.2255 g in 5 mL of EG) were successively added into a 100 mL flask placed in an oil bath at room temperature. The solution was moderately stirred for 30 min, and then slowly heated to 180 °C in 10–20 min. Nitrogen gas (150 mL min−1) was bubbled through the reaction during heating. Once the temperature reached 180 °C, the solution was naturally cooled to a certain temperature, such as 150–170 °C without stirring. After reaction for a period, the solution was cooled to room temperature.
For impurity level analysis, the final synthetic solution was washed by addition of the same volume of water and high speed centrifugation to remove excess PVP and other reagents, and the deposit was vacuum-dried and weighed as the total amount of the product. AgNWs alone in the final product were obtained by treating the final synthetic solution with a large amount of NH3·H2O to remove large NPs (AgCl and AgBr) and with acetone to remove small Ag NPs,16 and vacuum drying.
AgNW TCFs were coated using an automatic coating machine (BEVS 1811/2) equipped with an OSP-22 scraper, where the coating rate was fixed at 100 mm s−1. After drawing the preformed aqueous AgNW ink on a polyethylene terephthalate (PET) substrate, the start button was pressed down and the TCFs were coated and finally fabricated after a brief annealing process at 60 °C for 5 min.
The morphologies of the AgNWs were characterized by scanning electron microscopy (SEM) (Sirion 200 FEG) and transmission electron microscopy (TEM) (JEM-2010 transmission electron microscope). The composition was analyzed by energy dispersive X-ray analysis (EDX) (Oxford Instruments Analytical Limited). Optical transmittance spectra were obtained on a UV-vis-NIR spectrometer (Shimadzu UV-3600). The sheet resistance of the AgNW TCFs was measured using a four-point probe method (RST-9, Four-Probe Tech.).
Results and discussion
SEM images in Fig. 1 exhibit that the AgNWs synthesized at a constant temperature of 180 °C in the presence of high concentrations of Cl− and Br− ions are indeed thin and long, with an average diameter below 25 nm and an average length above 20 μm. However, the as-synthesized product contains numerous NPs, with one set of large particles in the size range of 200–500 nm, and another set of small particles 20–80 nm in size. Initially, upon mixing of Ag+ with Cl− and Br− in the precursor, large particles composed of Ag, Cl, and Br were produced at room temperature. As demonstrated in Fig. S1, ESI,† the NPs are enriched with Ag, and are possibly a composite of AgCl, AgBr and Ag. When the precursor solution was heated to 180 °C in 13 min, and held at this temperature for different time durations, the AgNWs nucleated and grew longitudinally, and the large particles gradually decreased in size and population. At an intermediate time (say, 40 min), small Ag NPs appeared in a big population. After 1 h, there remained few large particles, and VT-AgNWs were accompanied by huge amounts of small Ag NPs (Fig. 1(d)), mostly MTPs (Fig. S2, ESI†).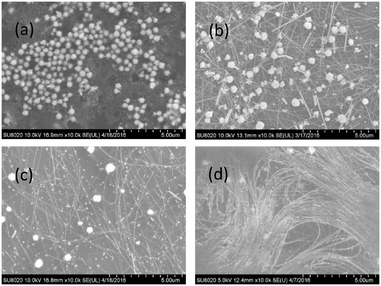 | ||
| Fig. 1 SEM images of AgNWs synthesized at a constant temperature of 180 °C for different time durations: (a) 0 min, (b) 20 min, (c) 40 min, (d) 60 min. | ||
These Ag NPs greatly increased the process complexity in post-purification runs. Especially when the production is scaled up, the method reported by Li et al. will be less efficient.16 Considering that small Ag NPs appeared after a certain time duration, they most possibly originated from secondary seeding by homogeneous nucleation in solution. As suggested in the literature,19,24 the energy gap between nucleation on heterogeneous sites of silver halides and homogeneous sites in solution favors the former one at first, and both are stimulated after acetaldehyde accumulation with time. Because homogeneous nucleation is favored at a higher temperature than the growth of AgNWs,17,19,24,25 reducing the process temperature from 180 °C prior to the burst of homogeneous nucleation may suppress secondary seeding, hence producing VT-AgNWs with improved purity. We first checked the process temperatures of 140, 150, 160, and 170 °C, and representative SEM images are exhibited in Fig. S3, ESI.† At 170 °C, the amount of small NPs lessened dramatically. It decreased further at 160 °C. The diameter and length of VT-AgNWs did not vary much. When the temperature decreased to 150 °C, some thick and short nanorods appeared. Upon further decreasing the temperature to 140 °C, no AgNWs were produced at all, which implies that 140 °C is the lower bound for nanowire growth in this synthetic system. So far, the optimal conditions to produce VT-AgNWs with excellent structural characteristics and much improved purity have been demonstrated by first heating the precursor solution containing large NPs to 180 °C, and then cooling it to 160 °C followed by holding at this temperature for 2 h (Fig. 2). TEM characterization in Fig. 3 shows that the VT-AgNWs have uniform morphology and a narrow dispersion in diameter with an average value of ∼21 nm. Combining with the average length of ∼34 μm from SEM analysis as shown in Fig. S4, ESI,† an aspect ratio larger than 1500 has been achieved.
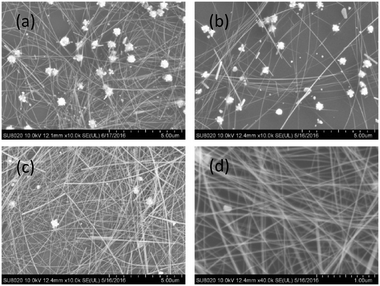 | ||
| Fig. 2 SEM images of AgNWs synthesized by first heating to 180 °C and then cooling to 160 °C with different holding times: (a) 40 min, (b) 60 min, (c) 120 min. (d) High-magnification view of (c). | ||
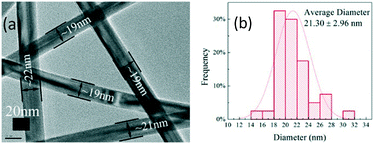 | ||
| Fig. 3 TEM characterization of VT-AgNWs synthesized by first heating to 180 °C and then cooling to 160 °C with a holding time of 2 h: (a) TEM image, (b) statistical diagram of diameter distribution. | ||
Optical absorption characterization could also provide key clues to the structural evolution of the products. We examined the samples under the process temperature of 140 °C for different holding times. As shown in Fig. 4(a), a broad peak initially appeared at 436 nm, corresponding to a plasmon peak of Ag NPs.26 The peak position red-shifted with the holding time, indicating the growth of NPs. However, as mentioned previously, no AgNWs grew at 140 °C, which is also reflected from the spectra as no absorption peaks characteristic of AgNWs have been detected. When the process temperature was 160 °C, as shown in Fig. 4(b), the intensity of the peak from Ag NPs declined with time, whereas peaks at 350–380 nm characteristic of AgNWs appeared and gained intensity with time, which means that AgNWs are dominant in the final product.
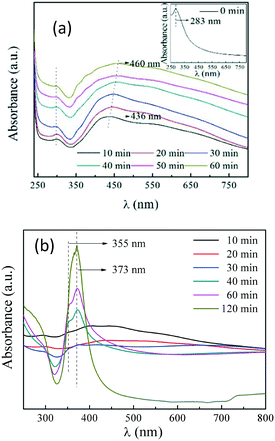 | ||
| Fig. 4 Optical absorption spectra of the products synthesized by first heating to 180 °C and then cooling to (a) 140 °C and (b) 160 °C with different holding times, respectively. | ||
Making use of time delay between heterogeneous and homogeneous nucleation through intentional temperature variation contributes to the high purity of VT-AgNWs. One may wonder whether it is possible to achieve this goal by simply applying a lower reaction temperature throughout the whole process. Temperatures of 140, 150, 160, and 170 °C were tested and the results are shown in Fig. S5, ESI.† Reaction at 140 °C led to large NPs without AgNWs. When the temperature was raised from 150 to 170 °C, AgNWs appeared with small NPs increasing in amount and large NPs conversely. The weight percentage of impurities in the final product is listed in Table 1. We can decrease the percent of NPs to 5.5%, much lower than the reported value of 42.3%.16 It is clear that preheating to a high temperature (180 °C) is a prerequisite for the removal of large NPs.
| Synthesis conditions | Impurity particle percentage | References |
|---|---|---|
| Heated to 180 °C, then cooled to 160 °C, and held for 2 h | 5.5% | This work |
| Heated to 160 °C, and held for 2 h | 27.2% | This work |
| Heated to 170 °C, and held for 1 h | 42.3% | Ref. 16 |
| Heated to 160 °C, and held for 35 min (slow injection of AgNO3) | 15% | Ref. 30 |
Schuette and Buhro proposed that the Ag source for AgNWs was mainly from the solution (that is, the AgNO3 precursor), instead of the large NPs.24,25 We wonder whether the degradation of the preformed large silver halide particles made any impact on the growth of VT-AgNWs. Considering that large NPs, either AgCl or AgBr, might degrade faster in the presence of H2O, we intentionally added 1 mL of de-ionized water for 30 min of reaction at 160 °C. From Fig. S6, ESI,† it is readily seen that with the addition of H2O, the population of large NPs decreased dramatically; however, numerous small NPs appeared, accompanied by some short and thick nanorods. We conclude that for the growth stage, the Ag source for the AgNWs was mainly from the solution, while for the heterogeneous nucleation and MTP formation stage, Ag from AgCl and AgBr did contribute somewhat. On the basis of this conclusion, three key events at different temperatures occur as follows: disintegration of AgCl and AgBr NPs at 180 °C or higher, heterogeneous nucleation at 160 °C or higher, and surface catalyzed Ag+ reduction for AgNW growth at 150 °C or higher. The reaction process for our optimized conditions could be depicted as follows: preheating to 180 °C helped large NPs formed upon room temperature mixing disintegrate, accompanied by heterogeneous nucleation at partially disintegrated sites, and holding at 160 °C drove surface catalyzed Ag+ reduction on heterogeneous seeds for AgNW growth, followed by successive heterogeneous nucleation and AgNW growth until the depletion of large NPs, during which homogeneous nucleation was suppressed simultaneously. When H2O was introduced, the disintegration and heterogeneous nucleation rates were accelerated, so large numbers of seeds appeared in short time which limited the growth of all seeds into MTPs with some of them evolving into single crystals, and at the same time, some MTPs might fluctuate to grow into nanorods. The reasons why water affected the nucleation rate are as follows. The problem on low solubility is usually faced by reduction of metal cations in non-aqueous solvents, so it is expected that transfer of electrons between reacting species could be facilitated when water increases the polarity of the ethylene glycol solvent.27 Moreover, a trace amount of water could promote proton hopping on the surface of the materials,28 and enhance the Ostwald ripening process;29 therefore, the disintegration of large Ag halide particles for Ag nucleation and Ostwald ripening was accelerated with water. Thick nanorods were also observed for the low process temperature of 150 °C (Fig. S3(b), ESI†). At this temperature, the heterogeneous nucleation rate was low until accumulation of enough acetaldehyde, but the time delay caused fluctuations in the growth of MTPs and the low temperature increased the critical nucleation radius, both of which were responsible for the nanorod formation. When further decreasing the temperature to 140 °C, surface catalyzed Ag+ reduction could not proceed and there were no AgNWs at all (Fig. S3(a) and S5(a), ESI†). The two situations under 140 °C are a little different. For the constant 140 °C reaction as shown in Fig. S5(a), ESI,† no seeds were created, while in Fig. S3(a), ESI,† heterogeneous nucleation initiated at 180 °C but was hindered at 140 °C, then further heating to 160 °C could activate AgNW growth from the existing seeds, which was confirmed as shown in Fig. S7, ESI.† Concerning the constant temperature reaction at 150–170 °C, large AgCl and AgBr NPs were not well-disintegrated and survived to the end of the synthesis process, while homogeneous nucleation proceeded to produce small Ag NPs. This temperature-dependent dominating process shifting from partial disintegration to homogeneous nucleation led to the synthesized products with more large NPs or small NPs as shown in Fig. S5, ESI.†
As the concentration of halide ions has a significant impact on the population of Ag NPs and the diameter of AgNWs,16,17,19 we further studied the impact under different kinds and concentrations of halide ions. First, when the concentration of Br− ions was reduced from our initially used value as suggested by Li et al.16 to 80% while keeping that of Cl− ions unchanged, VT-AgNWs with an average diameter of ∼22 nm and an average length of ∼29 μm could still be synthesized within a much shorter time, say, 1 h instead of 2 h used before. As expected, the population of Ag NPs in the product was even lower (Fig. S8 and S9, ESI†). Second, is it possible to use only Br− ions to produce thinner AgNWs? Actually, Silva et al. synthesized AgNWs with a similar diameter of 20 nm using only Br− ions, through slow injection of AgNO3 into the reaction solution as well as short time termination of the reaction.30 We applied our temperature variation method to synthesize AgNWs using only Br− ions and the results are shown in Fig. 5 and S10, ESI.† It was found that, for complete removal of AgBr precursor particles, 2 h of reaction at 160 °C after lowering from 180 °C resulted in thick Ag nanowires with an average diameter of ∼27 nm when the concentration of Br− ions was not very high; when the concentration was tripled, thin AgNWs with an average diameter of ∼21 nm could be obtained, but the enhanced etching ability of Br− ions resulted in etching of lots of pentagonally twinned Ag seeds,18,30 which could not grow into nanowires, and huge amounts of nanoparticle by-products were observed. Briefly, there is not enough room for further decrease of the AgNW diameter using only Br− ions, due to their excessive nucleation rate and enhanced etching ability which are not suitable for long time growth of AgNWs into very long wires. A new technique has to be exploited for the synthesis of high purity AgNWs with a diameter below 20 nm.
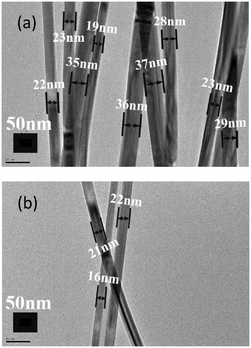 | ||
| Fig. 5 TEM characterization of AgNWs synthesized using only bromine ions at low (a) and high (b) concentration. | ||
Finally, the synthesized AgNWs were simply washed with water and formulated into an aqueous ink with HPMC, Sago-dispersant and Sago-flatting agent for coating of TCFs. The optical and electrical properties of films made of high purity AgNWs as well as those fabricated by the traditional heating method are compared in Fig. 6 and S11, ESI† and are summarized in Table 2. TCFs made of high purity AgNWs having a sheet resistance of 59 Ω sq−1 exhibited even better optical properties than those made from traditional AgNWs having a sheet resistance of 150 Ω sq−1. Transmittance (T%) was increased from 87.9% to a superior value of 91.7%; meanwhile, the haze (H%) was decreased from 4.2% to 1.6%, indicating that abundant NPs existed in the traditional AgNWs, scattered incident light and deteriorated the optical properties. Moreover, high purity AgNWs with few NPs guaranteed the uniformity of TCFs as the sheet resistance non-uniformity factor (NUF) was decreased from 9.7% to 6.7%, which is beneficial for the sensitivity and accuracy of touch panels.31 It should be pointed out that the optical values were obtained from the entire film with the underlying substrate of PET, which has more reference value for real applications. By further removal of the remaining impurity and applying an additional optical adjustment layer into the films of AgNWs/PET, which will be studied separately in depth, the haze value could be reduced once more to that of high quality indium tin oxide (ITO), satisfying the needs of high-definition applications.
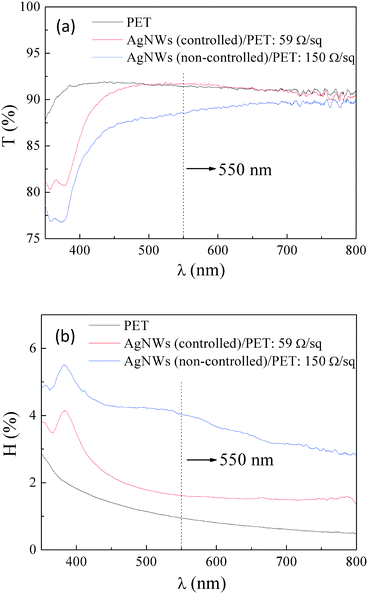 | ||
| Fig. 6 Visible light transmittance (a) and haze (b) of TCFs made of AgNWs with a temperature-controlled or non-controlled process, along with those of underlying PET substrates. | ||
| Materials | Transmittance (%) | Haze (%) | Sheet resistance (Ω sq−1) | Non-uniformity factor (%) |
|---|---|---|---|---|
| AgNWs (controlled)/PET | 91.73 | 1.62 | 59.0 | 6.65 |
| AgNWs (non-controlled)/PET | 87.92 | 4.22 | 150.0 | 9.70 |
| PET | 90.73 | 0.95 | — | — |
In future work, effort will be devoted to elucidating the exact transformation process of large NPs and how they contribute to the growth of VT-AgNWs. Although the NP percentage was remarkably reduced from the reported value of 42.3% to 5.5% in this work, the overall yield of VT-AgNWs is still below 50%, slightly enhanced from the value determined by Li et al. (33.8%).16 This finding may indicate that many of the Ag+ ions remained unreduced. Simply lengthening the reaction time did not work properly, because the amount of Ag NPs increased. Therefore, a method to incorporate more Ag+ ions into the growing VT-AgNWs as well as to effectively suppress the secondary seeding event has to be developed. Further work is currently under way in our laboratory.
Conclusions
In summary, we have synthesized very thin Ag nanowires with an average diameter of 21 nm and an average length of 34 μm. The purity of the product was improved dramatically by suppressing homogeneous nucleation at the growth stage. The ability to synthesize VT-AgNWs with high purity will make post-purification more scalable, and provide this material with more opportunities for high-end touch panel applications in next-generation portable electronic devices.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11274308, 51501182, 51502295 and U1532268), and the CAS/SAFEA International Partnership Program for Creative Research Teams.Notes and references
- L. B. Hu, H. Wu and Y. Cui, MRS Bull., 2011, 36, 760 CrossRef CAS.
- V. Scardaci, R. Coull, P. E. Lyons, D. Rickard and J. N. Coleman, Small, 2011, 7, 2621 CrossRef CAS PubMed.
- P. Lee, J. Lee, H. Lee, J. Yeo, S. Hong, K. H. Nam, D. Lee, S. S. Lee and S. H. Ko, Adv. Mater., 2012, 24, 3326 CrossRef CAS PubMed.
- T. Araki, J. T. Jiu, M. Nogi, H. Koga, S. Nagao, T. Sugahara and K. Suganuma, Nano Res., 2014, 7, 236 CrossRef CAS.
- C. H. Chung, T. B. Song, B. Bob, R. Zhu and Y. Yang, Nano Res., 2012, 5, 805 CrossRef CAS.
- A. R. Madaria, A. Kumar and C. W. Zhou, Nanotechnology, 2011, 22, 245201 CrossRef PubMed.
- R. M. Mutiso, M. C. Sherrott, A. R. Rathmell, B. J. Wiley and K. I. Winey, ACS Nano, 2013, 7, 7654 CrossRef CAS PubMed.
- S. Sorel, P. E. Lyons, S. De, J. C. Dickerson and J. N. Coleman, Nanotechnology, 2012, 23, 185201 CrossRef PubMed.
- G. Khanarian, J. Joo, X. Q. Liu, P. Eastman, D. Werner, K. O'Connell and P. Trefonas, J. Appl. Phys., 2013, 114, 024302 CrossRef.
- S. M. Bergin, Y. H. Chen, A. R. Rathmell, P. Charbonneau, Z. Y. Li and B. J. Wiley, Nanoscale, 2012, 4, 1996 RSC.
- Y. X. Ran, W. W. He, K. Wang, S. L. Ji and C. H. Ye, Chem. Commun., 2014, 50, 14877 RSC.
- S. Sorel, P. E. Lyons, S. De, J. C. Dickerson and J. N. Coleman, Nanotechnology, 2012, 23, 185201 CrossRef PubMed.
- M.-S. Lee, K. Lee, S.-Y. Kim, H. Lee, J. Park, K.-H. Choi, H.-K. Kim, D.-G. Kim, D.-Y. Lee, S. Nam and J.-U. Park, Nano Lett., 2013, 13, 2814 CrossRef CAS PubMed.
- E.-J. Lee, M.-H. Chang, Y.-S. Kim and J.-Y. Kim, APL Mater., 2013, 1, 042118 CrossRef.
- E.-J. Lee, Y.-H. Kim, D. K. Hwang, W. K. Choi and J.-Y. Kim, RSC Adv., 2016, 6, 11702 RSC.
- B. Li, S. R. Ye, I. E. Stewart, S. Alvarez and B. J. Wiley, Nano Lett., 2015, 15, 6722 CrossRef CAS PubMed.
- K. L. Zhang, Y. G. Du and S. M. Chen, Org. Electron., 2015, 26, 380 CrossRef CAS.
- F. Wu, W. H. Wang, Z. F. Xu and F. L. Li, Sci. Rep., 2015, 5, 10772 CrossRef PubMed.
- K. L. Zhang, Y. G. Du and S. M. Chen, J. Nanosci. Nanotechnol., 2016, 16, 480 CrossRef CAS.
- L. B. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4, 2955 CrossRef CAS PubMed.
- B. Wiley, Y. Sun and Y. Xia, Acc. Chem. Res., 2007, 40, 1067 CrossRef CAS PubMed.
- S. H. Im, Y. T. Lee, B. Wiley and Y. N. Xia, Angew. Chem., Int. Ed., 2005, 44, 2154 CrossRef CAS PubMed.
- C. H. An, J. Z. Wang, S. T. Wang, Q. H. Zhang, M. Yang and J. H. Zhan, CrystEngComm, 2012, 14, 5886 RSC.
- W. M. Schuette and W. E. Buhro, ACS Nano, 2013, 7, 3844 CrossRef CAS PubMed.
- W. M. Schuette and W. E. Buhro, Chem. Mater., 2014, 26, 6410 CrossRef CAS.
- Y. G. Sun, Y. D. Yin, B. T. Mayers, T. Herricks and Y. N. Xia, Chem. Mater., 2002, 14, 4736 CrossRef CAS.
- C. Yang, Y. Tang, Z. Su, Z. Zhang and C. Fang, J. Mater. Sci. Technol., 2015, 31, 16 Search PubMed.
- L. R. Merte, G. Peng, R. Bechstein, F. Rieboldt, C. A. Farberow, L. C. Grabow, W. Kudernatsch, S. Wendt, E. Laegsgaard, M. Mavrikakis and F. Besenbacher, Science, 2012, 336, 889e893 CrossRef PubMed.
- E. Matijevic, Chem. Mater., 1993, 5, 412 CrossRef CAS.
- R. R. Silva, M. X. Yang, S.-Il. Choi, M. F. Chi, M. Luo, C. Zhang, Z.-Y. Li, P. H. C. Camargo, S. J. L. Ribeiro and Y. N. Xia, ACS Nano, 2016, 10, 7892 CrossRef PubMed.
- Y. Jia, C. Chen, D. Jia, S. Li, S. Ji and C. Ye, ACS Appl. Mater. Interfaces, 2016, 8, 9865 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: EDX spectrum, additional SEM images and size statistics of the synthesized Ag products. See DOI: 10.1039/c6ce02075e |
| This journal is © The Royal Society of Chemistry 2017 |
