In situ crystallization study of impurity phases in Bi–Fe–O thin films grown by atomic layer deposition
Andrew R.
Akbashev
,
Matthias
Falmbigl
,
Aleksandr V.
Plokhikh
and
Jonathan E.
Spanier
*
Department of Materials Science & Engineering, Philadelphia, PA, USA. E-mail: spanier@drexel.edu
First published on 1st December 2016
Abstract
We report on the phase evolution of pure and Fe-doped bismuth oxide (Bi–O and Bi–Fe–O) thin films grown by atomic layer deposition. In situ X-ray diffraction was used to map the evolution of phase formation and orientational growth of the oxides during the gradual crystallization of the films from the amorphous state on single-crystalline substrates. The formation of (001)-oriented Bi2O2.3 was observed at temperatures as low as 300 °C on (001)SrTiO3, with a gradual transformation into Bi2O3. A similar crystallization of Fe-doped Bi2O3 on (111)ZrO2(Y2O3) leads to the formation of (111)-oriented δ-Bi2O3 above 400 °C instead of the sillenite phase. We thus demonstrate that epitaxial stabilization of the metastable phases can take place during atomic layer deposition of oxides as well as post-deposition annealing. In situ crystallization of the amorphous Bi–Fe–O films revealed the evolution of oxide phases in the film with the sillenite composition. Our results are important for the design of annealing procedures to obtain phase-pure epitaxial BiFeO3 thin films from amorphous stoichiometric Bi–Fe–O grown by atomic layer deposition.
1 Introduction
The growth of high-quality epitaxial thin films of BiFeO3, one of the most promising multiferroics, has long been an important milestone for practical technology based on the intrinsic coupling of ferroelectric and magnetic ordering. High-quality epitaxial BiFeO3 thin films have been grown using pulsed laser deposition (PLD),1 metal–organic chemical vapor deposition (MOCVD),2,3 molecular beam epitaxy (MBE),4 and other high-temperature techniques. Recently, atomic layer deposition (ALD) has been shown to be a viable route for producing high-quality BiFeO3 thin films via a two-step process (low-temperature ALD growth and post-deposition annealing).5–7 ALD is a highly versatile method for obtaining thin films and coatings,8 which offers an inexpensive and scalable alternative to physical vapor deposition. Because of the phase composition complexity in Bi–Fe–O films, one of the major difficulties in the BiFeO3 film production is the formation of undesirable secondary phases. Bi–Fe–O film phase composition has been reported for PLD and MOCVD, where deposition is carried out at high temperatures and ex situ annealing is not needed for crystallization. However, in the case of ALD-grown Bi–Fe–O a proper thermal processing requires detailed study of the secondary phase crystallization. One of the parasitic phases that poses a major challenge during Bi–Fe–O crystallization is the sillenite phase Bi26−xFexO39, which is isostructural to γ-Bi2O3 and appears both in the synthesis of thin film and ceramics.9Here we investigate the appearance of parasitic Bi-rich phases during crystallization of the ALD-grown Bi–Fe–O films on single-crystalline (001)SrTiO3 and (111)ZrO2(Y2O3) (YSZ). First, we performed an in situ study of the crystallization of the pure bismuth oxide thin film. Then, these results are used to interpret the crystallization of a more complex Fe-doped bismuth oxide film.
2 Experimental methods
ALD of Bi–Fe–O thin films was carried out using an ALD reactor (Cambridge Nanotech Savannah 100) on single-crystalline SrTiO3 and YSZ substrates (MTI Corporation) following ALD recipes described in our previous work.5,11 Substrates were placed ∼3–4 cm from the gas inlet and the chamber was held at 200 °C during growth. Ferrocene (Fe(cp)2, Sigma-Aldrich F408) and Bi(mmp)3 (tris(1-methoxy-2-methyl-2-propoxy)bismuth, Sigma-Aldrich American Elements, PN: BI-OMX-03M-C, 99.9% purity) were heated to 90 °C and 135–145 °C, respectively, and used as volatile precursors. Oxidation of each precursor layer was carried out using ozone (O3). Growth of the films with considerable Bi excess (sillenite composition) was achieved with a smaller Fe(cp)2 vapor pressure in the evaporator, which in turn led to a smaller Fe delivery to the film.X-ray diffraction was performed in a 4-circle X-ray diffractometer (Rigaku Smartlab, 40 kV, 44 mA, Cu Kα) equipped with a double (220)Ge monochromator in a parallel beam geometry. In situ XRD measurements took place in a commercial domed hot stage (Anton Paar DHS 1100) in low vacuum (10−1 mbar) with a temperature step of 25 °C and 40 min of the total measurement time at each temperature point; the heating rate was ∼5 °C min−1. The intensity of the sillenite peak on (111)YSZ was averaged over the range of 0.1° around the peak maximum to reduce the noise and then the intensity-versus-temperature graph was plotted.
3 Results and discussion
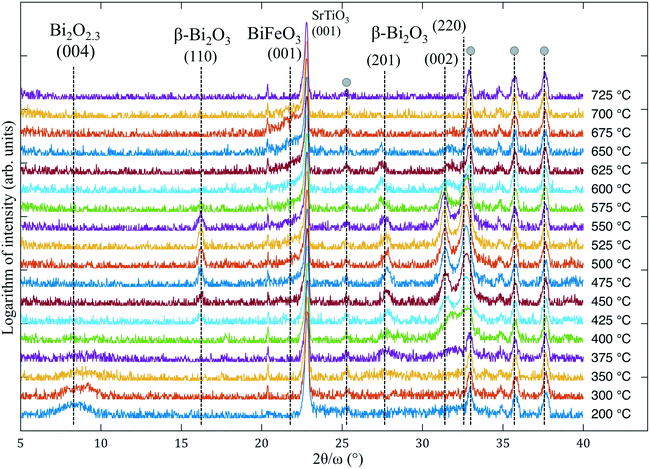
A low growth temperature (250 °C) does not initiate the crystallization of pure ALD-grown Fe–O, but leads to the amorphous state of the film. However, the ALD of Bi–O at ∼250–300 °C typically results in a partially crystalline film.5 The latter may affect the crystallinity of a more complex film composition that contains Bi–O (such as BiFeO3 or Bi4Ti3O12 films12), especially if the ALD growth is carried out above 300 °C.Results of the in situ X-ray diffraction (XRD) of ALD-grown Bi–O crystallization are shown in Fig. 1. In the temperate range of 300–350 °C an intense peak at 2θ = 9.5° is observed, along with other weak reflections. Among numerous bismuth oxides there is only one phase that can produce a high-intensity XRD reflection at such low angles: Bi2O2.3, i.e. which is Bi6O7. This oxide has a tetragonal structure (I4/mmm) with a = 3.85 Å, matching well with the lattice constant of SrTiO3, and a very large c parameter of 35.1 Å.10 The Bi2O2.3 epitaxial phase appearance was reported13 in pure-phase films grown by CVD under a low oxygen partial pressure on cubic substrates (such as SrTiO3 and MgO) at 600 °C. In our case we assume that the appearance of Bi2O2.3 instead of Bi2O3 is due to the low-temperature ALD process that led to “under-oxidized” Bi–O thin films. This phase disappears above 350 °C and does not exist at 600 °C, as was reported for the CVD-grown Bi2O2.3. We propose several possible explanations for occurrence of crystallization at such a low temperature: (a) the Bi2O2.3 phase is lattice-matched to the substrate, which can effectively lower the crystallization temperature of Bi2O2.3; (b) bismuth oxide in general is known to crystallize at temperatures close to 250 °C or lower;14 and (c) the as-grown Bi–O film is primarily amorphous and therefore has a very high Gibbs free energy, which makes it easy for the film to nucleate and grow in the form of epitaxial Bi2O2.3 nanocrystallites.
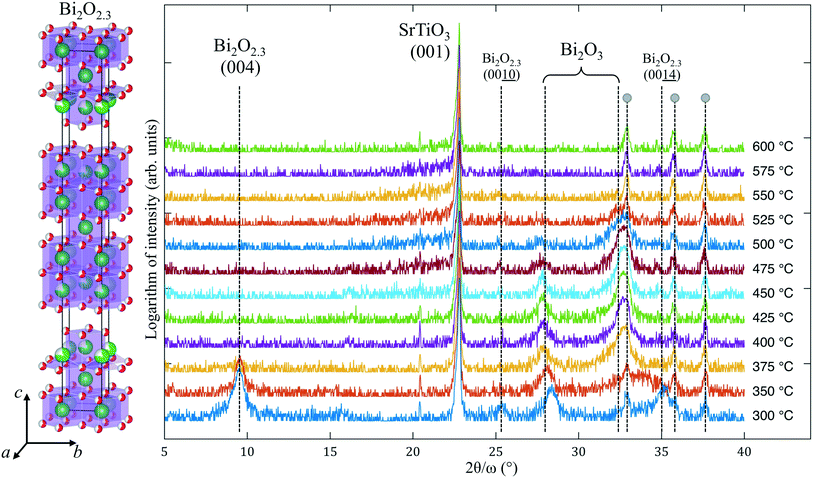 | ||
| Fig. 1 XRD patterns collected during in situ annealing of the Bi–O thin film on (001)SrTiO3. Large gray dots designate the peaks coming from the hot stage. The structure10 of Bi2O2.3 is shown on the left. | ||
Another factor that reflects the metastability of Bi2O2.3 is its transformation into a more stable bismuth oxide when the substrate temperature reaches 375 °C. Peaks at 2θ = 9.5° and 35.2° disappear, leaving the peak at 28.1° almost intact, and a new reflection at ∼32.7° emerges. Under heating to 700 °C, these two peaks persist up to ∼500 °C and disappear at higher temperature without any new X-ray reflections showing up. Energy-dispersive X-ray spectroscopic (EDS) analysis of the films after the in situ annealing shows no traces of bismuth, i.e., it evaporated completely at T > 550 °C. Because the peaks at 28.1° and 32.7° may be ascribed to different Bi2O3 phases, it is unclear into which phase Bi2O2.3 transformed. For example, it was reported that magnetron-sputtered Bi–O films grown at 200 °C contained δ-Bi2O3, considered to be a high-temperature polymorph, but after a slight change in the growth temperature (to 250 °C) a mixture of α-Bi2O3 and β-Bi2O3 formed.15 The same films at 150 °C were amorphous. Interestingly, ALD-grown Bi2O3 was reported to be α-Bi2O3 (on Si(100)) above 250 °C with a transformation into γ-Bi2O3 upon annealing at ∼600 °C,16 but films deposited at 90–150 °C showed the presence of presumably β-Bi2O3 ([201]-oriented),17 which clearly changed into a polycrystalline film above 270 °C. Although the peak at 28.1° can be interpreted as the reflection of the (111)δ-Bi2O3, the appearance of this polymorph is highly unlikely due to (a) a narrow temperature window of its stability, (b) pronounced lattice parameter mismatch with the SrTiO3 substrate, and (c) the fact that cubic (111)δ-Bi2O3 cannot be epitaxially accommodated on (001)SrTiO3 and such (111)-facet growth is energetically unfavorable on the (001) substrates.
Studying the Bi–O crystallization is necessary to understand the phase evolution in non-stoichiometric Bi–Fe–O films. The in situ XRD crystallization of the Bi–Fe–O film is shown in Fig. 2. EDS analysis of the sample reveals the starting composition as Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe = 9
Fe = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (which was expected to produce primarily a sillenite phase) and the final composition as Bi ≪ Fe. The as-grown films show the presence of essentially one broad peak at ∼8.5°, which we have already identified as the Bi2O2.3 phase in the case of the Bi–O films. As in the previous experiment, this phase disappears at 375 °C and several other peaks emerge above ∼400 °C. The first peak at 2θ = 16.2° can correspond either to the (001) reflection of Bi2Fe4O9,18 which is ruled out on the following basis: (a) this phase is thermodynamically stable only in the case of Bi
1 (which was expected to produce primarily a sillenite phase) and the final composition as Bi ≪ Fe. The as-grown films show the presence of essentially one broad peak at ∼8.5°, which we have already identified as the Bi2O2.3 phase in the case of the Bi–O films. As in the previous experiment, this phase disappears at 375 °C and several other peaks emerge above ∼400 °C. The first peak at 2θ = 16.2° can correspond either to the (001) reflection of Bi2Fe4O9,18 which is ruled out on the following basis: (a) this phase is thermodynamically stable only in the case of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe < 1 according to the Bi2O3–Fe2O3 phase diagram,19 and (b) no (002) reflection at 2θ = 29.7° is present. Thus, we suggest that the peak at 16.2° corresponds to (110)β-Bi2O3 (possibly doped by Fe).20 The next peaks with 2θ = 27.8°, 31.5° and 32.8° exist between 375 °C and 650 °C and their location points to the β-Bi2O3 phase as their origin (according to ref. 20). In (ref. 21) the formation of Bi2O3 occurred in the same 2θ range and was more pronounced with an increase in Bi content. The formation of the sillenite phase (isostructural to γ-Bi2O3) instead of or along with β-Bi2O3 is also possible since the two phases share similar peak positions from 27° to 34°. It is important to note that, although there are no studies of Fe-doped β-Bi2O3, the doping of the β polymorph by other cations can be done and, as reported,20,22 it can improve the structural stability of otherwise metastable β-Bi2O3, which could take place in our case. In the range from 500 °C up to 700 °C a small fraction of the (001)-oriented BiFeO3 layer forms, as indicated by a shoulder on the right side of the substrate peak. Thus, the following scenario can be proposed: at lower temperatures (200–350 °C) the crystallization of Bi2O2.3 takes place, then β-Bi2O3 forms and exists up to almost 650 °C; meanwhile, at 500 °C amorphous Fe–O reacts with the bismuth oxide and forms BiFeO3. We note that the appearance of the sillenite phase (γ-Bi2O3) instead of β-Bi2O3 in the annealing process was initially expected. Instead, the formation of metastable polymorphs (Bi2O2.3 and β-Bi2O3) occurred, indicating the complexity of the crystallization from the amorphous films.
Fe < 1 according to the Bi2O3–Fe2O3 phase diagram,19 and (b) no (002) reflection at 2θ = 29.7° is present. Thus, we suggest that the peak at 16.2° corresponds to (110)β-Bi2O3 (possibly doped by Fe).20 The next peaks with 2θ = 27.8°, 31.5° and 32.8° exist between 375 °C and 650 °C and their location points to the β-Bi2O3 phase as their origin (according to ref. 20). In (ref. 21) the formation of Bi2O3 occurred in the same 2θ range and was more pronounced with an increase in Bi content. The formation of the sillenite phase (isostructural to γ-Bi2O3) instead of or along with β-Bi2O3 is also possible since the two phases share similar peak positions from 27° to 34°. It is important to note that, although there are no studies of Fe-doped β-Bi2O3, the doping of the β polymorph by other cations can be done and, as reported,20,22 it can improve the structural stability of otherwise metastable β-Bi2O3, which could take place in our case. In the range from 500 °C up to 700 °C a small fraction of the (001)-oriented BiFeO3 layer forms, as indicated by a shoulder on the right side of the substrate peak. Thus, the following scenario can be proposed: at lower temperatures (200–350 °C) the crystallization of Bi2O2.3 takes place, then β-Bi2O3 forms and exists up to almost 650 °C; meanwhile, at 500 °C amorphous Fe–O reacts with the bismuth oxide and forms BiFeO3. We note that the appearance of the sillenite phase (γ-Bi2O3) instead of β-Bi2O3 in the annealing process was initially expected. Instead, the formation of metastable polymorphs (Bi2O2.3 and β-Bi2O3) occurred, indicating the complexity of the crystallization from the amorphous films.
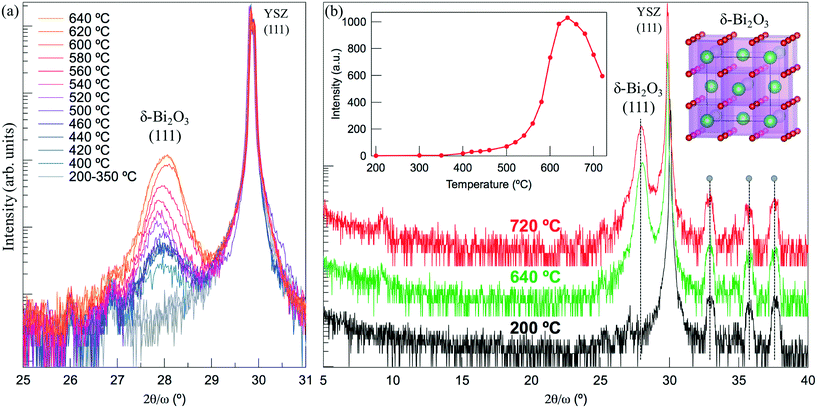
To study the crystallization of the sillenite phase on a different substrate, we performed a similar in situ XRD experiment for the Fe-doped Bi2O3 grown on (111)YSZ. This substrate has a fluorite crystal structure with a large lattice parameter (a = 5.125 Å) close to those of the sillenite (a = 10.18 Å, lattice mismatch is 0.6%). Fig. 3(a) shows the crystallization process, where we observed primarily one phase appearing during the in situ annealing of the Bi–Fe–O film (Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe = 4
Fe = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). However, this peak belongs to the (111)δ-Bi2O3 and not the (222)Bi26−xFexO39 (2θ = 30.39°). The growth of epitaxial (111)δ-Bi2O3 thin films was reported to be easily obtainable on (111)YSZ and (0001)Al2O3 substrates via physical vapor deposition.23,24 The crystallization begins at 400 °C and the peak increases up to ∼620–640 °C, after which the intensity starts to gradually decrease with temperature due to the bismuth oxide volatilization. The nature of the noisy low-intensity peak at 9° remains unclear. The inset in Fig. 3(b) shows the temperature evolution of the peak intensity. Importantly, no other phase shows up during crystallization, and the only peak available has a high intensity and a position that coincides with the diffraction angle from the (111)-oriented δ-Bi2O3. It is unclear why δ-Bi2O3 is observed instead of the sillenite phase (γ-Bi2O3). The following factors may contribute: (a) both YSZ and δ-Bi2O3 have a fluorite structure, which could serve as a basis for the solid-state epitaxial stabilization; and (b) δ-Bi2O3 should be able to accommodate Fe as a doping species, which can stabilize this phase at lower temperatures as happens in rare-earth-doped δ-Bi2O3.25 The difference in the crystallization of Fe-doped Bi2O3 on (001)SrTiO3 and (111)YSZ signifies the importance of lattice-matched substrate proximity for the epitaxial stabilization and growth of the oxides. We propose that the mechanism for the epitaxial crystallization of δ-Bi2O3 follows the same principle as in the case of the pure BiFeO3 crystallization on the lattice-matched substrate,5,11 where the crystallization front appears near the substrate-film interface and propagates epitaxially up to the surface of the film.
1). However, this peak belongs to the (111)δ-Bi2O3 and not the (222)Bi26−xFexO39 (2θ = 30.39°). The growth of epitaxial (111)δ-Bi2O3 thin films was reported to be easily obtainable on (111)YSZ and (0001)Al2O3 substrates via physical vapor deposition.23,24 The crystallization begins at 400 °C and the peak increases up to ∼620–640 °C, after which the intensity starts to gradually decrease with temperature due to the bismuth oxide volatilization. The nature of the noisy low-intensity peak at 9° remains unclear. The inset in Fig. 3(b) shows the temperature evolution of the peak intensity. Importantly, no other phase shows up during crystallization, and the only peak available has a high intensity and a position that coincides with the diffraction angle from the (111)-oriented δ-Bi2O3. It is unclear why δ-Bi2O3 is observed instead of the sillenite phase (γ-Bi2O3). The following factors may contribute: (a) both YSZ and δ-Bi2O3 have a fluorite structure, which could serve as a basis for the solid-state epitaxial stabilization; and (b) δ-Bi2O3 should be able to accommodate Fe as a doping species, which can stabilize this phase at lower temperatures as happens in rare-earth-doped δ-Bi2O3.25 The difference in the crystallization of Fe-doped Bi2O3 on (001)SrTiO3 and (111)YSZ signifies the importance of lattice-matched substrate proximity for the epitaxial stabilization and growth of the oxides. We propose that the mechanism for the epitaxial crystallization of δ-Bi2O3 follows the same principle as in the case of the pure BiFeO3 crystallization on the lattice-matched substrate,5,11 where the crystallization front appears near the substrate-film interface and propagates epitaxially up to the surface of the film.
4 Conclusions
We studied the crystallization of pure and Fe-doped bismuth oxide thin films on (001)SrTiO3 and (111)YSZ. An unusual metastable Bi2O2.3 phase appeared at low temperatures and persisted up to 350 °C. Instead of sillenite, which is regarded as a major parasitic phase during the synthesis of BiFeO3, we observed the crystallization of β-Bi2O3 in the temperature range from 400 to 600 °C on SrTiO3. The crystallization of Fe-doped Bi2O3 on (111)YSZ results in the epitaxial (111)δ-Bi2O3 (presumably Fe-doped) instead of the Bi26−xFexO39. Our findings imply that during annealing of ALD-grown amorphous oxides, the kinetically driven processes as well as the proximity to the lattice-matched substrate can play an important role in the phase content of the films. These results are paramount for choosing proper thermal treatment of ALD-grown Bi–Fe–O and subsequent synthesis of phase-pure epitaxial BiFeO3. Our studies demonstrate that during the ALD growth as well as the annealing of as-grown films, the formation of metastable oxides can take place via the epitaxial stabilization mechanism.Acknowledgements
We acknowledge the Drexel University core shared user facilities for access to XRD (NSF DMR 1040166). Work at Drexel was supported by ONR under No. N00014-15-11-2170 and also by NSF under No. DMR 1124696 and IIP 1403463.References
- N. Dix, R. Muralidharan, M. Varela, J. Fontcuberta and F. Sanchez, Appl. Phys. Lett., 2012, 100, 122905 Search PubMed.
- M. S. Kartavtseva, O. Y. Gorbenko, A. R. Kaul, A. R. Akbashev, T. V. Murzina, S. Fusil, A. Barthalamy and F. Pailloux, Surf. Coat. Technol., 2007, 201, 9149–9153 Search PubMed.
- J. Thery, C. Dubourdieu, T. Baron, C. Ternon, H. Roussel and F. Pierre, Chem. Vap. Deposition, 2007, 13, 232–238 Search PubMed.
- J. F. Ihlefeld, A. Kumar, V. Gopalan, D. G. Schlom, Y. B. Chen, X. Q. Pan, T. Heeg, J. Schubert, X. Ke, P. Schiffer, J. Orenstein, L. W. Martin, Y. H. Chu and R. Ramesh, Appl. Phys. Lett., 2007, 91, 071922 Search PubMed.
- A. R. Akbashev, G. Chen and J. E. Spanier, Nano Lett., 2013, 14, 44–49 Search PubMed.
- F. Zhang, G. Sun, W. Zhao, L. Wang, L. Zheng, S. Liu, B. Liu, L. Dong, X. Liu, G. Yan, L. Tian and Y. Zeng, J. Phys. Chem. C, 2013, 117, 24579–24585 Search PubMed.
- C. D. Pham, J. Chang, M. A. Zurbuchen and J. P. Chang, Chem. Mater., 2015, 27, 7282–7288 Search PubMed.
- V. Miikkulainen, M. Leskela, M. Ritala and R. L. Puurunen, J. Appl. Phys., 2013, 113, 021301 Search PubMed.
- A. Akbashev and A. Kaul, Russ. Chem. Rev., 2011, 80, 1159 Search PubMed.
- A. A. Zav‘yalova and R. M. Imamov, Sov. Phys. Crystallogr., 1968, 13, 37 Search PubMed.
- A. R. Akbashev, A. V. Plokhikh, D. Barbash, S. E. Lofland and J. E. Spanier, APL Mater., 2015, 3, 106102 Search PubMed.
- M. Vehkamäki, T. Hatanpää, M. Kemell, M. Ritala and M. Leskelä, Chem. Mater., 2006, 18, 3883–3888 Search PubMed.
- M. Schuisky and A. Harsta, Chem. Vap. Deposition, 1996, 2, 235–238 Search PubMed.
- D. Perez-Mezcua, R. Sirera, R. Jimenez, I. Bretos, C. De Dobbelaere, A. Hardy, M. K. Van Bael and M. L. Calzada, J. Mater. Chem. C, 2014, 2, 8750–8760 Search PubMed.
- H. T. Fan, S. S. Pan, X. M. Teng, C. Ye, G. H. Li and L. D. Zhang, Thin Solid Films, 2006, 513, 142–147 Search PubMed.
- Y. D. Shen, Y. W. Li, W. M. Li, J. Z. Zhang, Z. G. Hu and J. H. Chu, J. Phys. Chem. C, 2012, 116, 3449–3456 Search PubMed.
- D. Z. Austin, D. Allman, D. Price, S. Hose, M. Saly and J. F. Conley, J. Vac. Sci. Technol., A, 2014, 32, 01A113 Search PubMed.
- A. G. Tutov and V. N. Markin, Izv. Akad. Nauk SSSR, Neorg. Mater., 1970, 6, 2014–2017 Search PubMed.
- R. Palai, R. S. Katiyar, H. Schmid, P. Tissot, S. J. Clark, J. Robertson, S. A. T. Redfern, G. Catalan and J. F. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 014110 Search PubMed.
- C. Jovalekic, M. Zdujic, D. Poleti, L. Karanovic and M. Mitric, J. Solid State Chem., 2008, 181, 1321–1329 CrossRef CAS.
- N. Deepak, P. Carolan, L. Keeney, P. F. Zhang, M. E. Pemble and R. W. Whatmore, Chem. Mater., 2015, 27, 6508–6515 Search PubMed.
- Y. Wang, Y. Wen, H. Ding and Y. Shan, J. Mater. Sci., 2010, 45, 1385–1392 Search PubMed.
- P. Lunca Popa, S. Sønderby, S. Kerdsongpanya, J. Lu, N. Bonanos and P. Eklund, J. Appl. Phys., 2013, 113, 046101 Search PubMed.
- A. Gutiérrez-Llorente, H. Joress, A. Woll, M. E. Holtz, M. J. Ward, M. C. Sullivan, D. A. Muller and J. D. Brock, APL Mater., 2015, 3, 036105 Search PubMed.
- R. Punn, A. M. Feteira, D. C. Sinclair and C. Greaves, J. Am. Chem. Soc., 2006, 128, 15386–15387 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |