Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Prebiotic synthesis of 2-deoxy-D-ribose from interstellar building blocks promoted by amino esters or amino nitriles†
Andrew M.
Steer
,
Nicolas
Bia
,
David K.
Smith
 and
Paul A.
Clarke
and
Paul A.
Clarke
 *
*
Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: paul.clarke@york.ac.uk
First published on 8th September 2017
Abstract
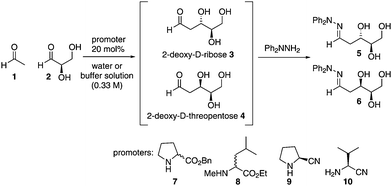
Understanding the prebiotic genesis of 2-deoxy-D-ribose, which forms the backbone of DNA, is of crucial importance to unravelling the origins of life, yet remains open to debate. Here we demonstrate that 20 mol% of proteinogenic amino esters promote the selective formation of 2-deoxy-D-ribose over 2-deoxy-D-threopentose in combined yields of ≥4%. We also demonstrate the first aldol reaction promoted by prebiotically-relevant proteinogenic amino nitriles (20 mol%) for the enantioselective synthesis of D-glyceraldehyde with 6% ee, and its subsequent conversion into 2-deoxy-D-ribose in yields of ≥ 5%. Finally, we explore the combination of these two steps in a one-pot process using 20 mol% of an amino ester or amino nitrile promoter. It is hence demonstrated that three interstellar starting materials, when mixed together with an appropriate promoter, can directly lead to the formation of a mixture of higher carbohydrates, including 2-deoxy-D-ribose.
It has previously been shown that nucleotides1–4 and D-tetroses5 can be formed under plausible prebiotic conditions. However, the origin of 2-deoxy-D-ribose (prebiotic or biotic), is open to debate6–9 – no completely satisfactory explanation has been put forward. Until recently it was assumed that carbohydrates were formed prebiotically via the formose reaction.10–12 Originally reported in 1861,10 the Ca(OH)2 promoted polymerisation of formaldehyde leads to a complex “sweet tasting” mixture of products, with a ribose component of <1%.13 Borate14 and silicate15,16 minerals have been added to the formose reaction in an attempt to stabilise the pentose products, and this has met with some success.17
Previous studies on the prebiotic synthesis of carbohydrates from simple aldehydes such as acetaldehyde, glycolaldehyde, formaldehyde and glyceraldehyde, have focused on using amino acid catalysts.18,19 Amino acids may have originated on Earth or may have extra-terrestrial origins, possibly through meteoritic bombardment,20–22 and the slight enantiomeric excesses (ee) of these amino acids could have been amplified through methods such as eutectic concentration.23 The amino acid catalysed reactions studied to date have required stoichiometric amounts of “catalyst”, extended reaction times, and give only trace amounts of carbohydrate products in low stereochemical purity.18,24,25 Initial studies with amino acid promoted aldol reactions focused on the formation of tetroses.18,26 Pizzarello and Weber showed the dimerization of glycolaldehyde by α,α-L-disubstituted amino acids led to the formation of L-tetroses in <7% ee.18 Similar studies by Breslow with stoichiometric quantities of L-proteinogenic amino acids showed the formation of D-glyceraldehyde with similarly low ees.24,25 While these reactions take many days and the yields of the carbohydrate products are very low, a study using dipeptides as catalysts was shown to improve the reaction selectivity and the yield of tetroses.27 In related studies, Darbre showed that a zinc–proline complex catalysed an aqueous aldol reaction in which a cocktail of higher carbohydrates was produced, including ribose in a 19% GC yield,28,29 although the ee of the carbohydrates was not reported.
Our own studies in this area have previously shown that, unlike native amino acids, esters of L-proteinogenic amino acids are capable of promoting the efficient aldol dimerisation of glycolaldehyde to form D-tetroses under simple prebiotic conditions, in good yields, and with the highest enantioselectivities to date (up to 68% ee).5 Given the success of this reaction we were intrigued to see if these conditions could be used for the formation of higher carbohydrates. We chose to study the formation of 2-deoxy-D-pentoses as they can be formed by the aldol condensation of acetaldehyde with D-glyceraldehyde, both deemed prebiotic building blocks,30–32 in a reaction which can generate only two diastereomeric products in each enantiomeric series, thus simplifying analysis of the product mixture. Importantly, 2-deoxy-D-ribose is an essential carbohydrate in biology and there is intense debate on whether it arose prebiotically or through an evolutionary adaptation once life got started.8 We hoped that this work might provide insight into whether simple prebiotic generation of 2-deoxy-D-ribose was indeed feasible. Possible pathways for pentose formation on the early Earth have shown that these compounds can be formed from glycolaldehyde using additives such as zinc–proline, calcium, borate or molybdate minerals.17,28,33,34 Oro and Cox first attempted the prebiotic synthesis of 2-deoxyribose from glyceraldehyde and acetaldehyde. Using calcium oxide as a base they were able to identify the formation of 2-deoxyribose.35 Ritson and Sutherland have also shown, using photoredox chemistry, that ribose can be formed from glycolaldehyde and subsequently be reduced to 2-deoxyribose.4 These studies show how pentose sugars could be formed on the early Earth but do not address a key question; how did the stereochemical preference for D-pentoses arise?
In this paper we show that sub-stoichiometric quantities of L-amino acid esters or nitriles are capable of promoting the formation of isolable quantities of 2-deoxy-D-ribose from equimolar mixtures of reagents in only 24 hours under prebiotically plausible conditions. We chose these challenging reaction constraints, reasoning that if the reactions were successful at generating carbohydrates under these conditions, then they would certainly also be viable under other possible prebiotic scenarios involving greater amounts of promoter, different stoichiometries or greater lengths of time.
In order to isolate the products, reaction mixtures were treated with N,N-diphenyl hydrazine in order to trap any carbohydrates formed and facilitate their isolation and characterisation as the hydrazone. Authentic samples of all possible hydrazone products were prepared using unambiguous routes,36 and control trapping experiments with authentic 2-deoxy-D-ribose, 2-deoxy-L-ribose, 2-deoxy-D-threopentose, D-glyceraldehyde and L-glyceraldehyde were performed to prove that no scrambling of stereochemistry under the reaction or trapping conditions took place.36 With these essential preliminaries in place, we then investigated the aldol reaction of acetaldehyde 1 and D-glyceraldehyde 2 with L-amino esters 7 and 8 in unbuffered water as well as in phosphate buffered water at pH = 6.0 and 7.0 (Table 1).
| Entrya | Promoterb | pH | Ratio (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)c 6)c |
Yieldd5 + 6 (%) |
|---|---|---|---|---|
| a Each set of conditions were run 3 times. b 20 mol% loading. c As measured by integration of the azomethine proton of the trapped hydrazone by 500 MHz 1H NMR after column chromatography. Ratios are the mean of 3 reactions. The spread of the ratio values between the 3 runs was ±0.1 from the mean. d Isolated yield after column chromatography and preparative TLC, averaged over the 3 runs. | ||||
| 1 | None | 7.0 | — | 0 |
| 2 | L-7 | Unbuffered | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 |
| 3 | L-7 | 7.0 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 |
| 4 | L-7 | 6.0 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 |
| 5 | D-7 | 7.0 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 |
| 6 | L-8 | 7.0 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5 |
| 7 | L-8 | 6.0 | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5 |
| 8 | D-8 | 7.0 | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 |
| 9 | 9 | 7.0 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 |
| 10 | 9 | 6.0 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 |
| 11 | 10 | Unbuffered | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 |
| 12 | 10 | 7.0 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 |
As can be seen from Table 1, amino esters 7 and 8 were capable of catalyzing the formation of 2-deoxy-D-ribose but in the absence of amino ester the reaction did not occur. In all instances 2-deoxy-D-ribose 5 predominated over the diastereomeric 2-deoxy-D-threopentose 6. Running the reaction at different pH values did not affect the yield or ratio of the products (entries 2, 3 and 4), thus increasing the possibility of the reaction proceeding in a prebiotic aqueous environment. Interestingly 2-deoxy-D-ribose was also the major product when promoters with the D-configuration were used (entries 5 and 8), showing that the anti-stereochemistry of 2-deoxy-D-ribose is inherently preferred but is influenced to a small degree by the stereochemistry of the promoter (compare for example entries 3 and 5, or entries 6 and 8). The D-configuration of the products arises from the chirality of the D-glyceraldehyde used as a starting material, and control experiments showed that enantio-integrity of D-glyceraldehyde was not eroded under the reaction conditions. All of the reactions generated isolable amounts of the products. The other components of the reaction mixture were found to be unreacted starting materials, isolated as their hydrazones.
As there is some debate to the prebiotic nature of amino esters; we wanted to refine our study to include even more prebiotically plausible promoters. We therefore turned our attention to amino nitriles, which, together with acetaldehyde, glycolaldehyde and glyceraldehyde, are formed under prebiotic conditions in Sutherland's cyanosulfidic photoredox systems chemistry proposal.30 Furthermore, Kawasaki and co-workers have shown how amino nitriles can be synthesized from Strecker reactions and obtained in high ees through crystallization or attack of HCN to a specific imine crystal face.37,38 We were delighted to find that using 20 mol% of amino nitrile, as before, gave 2-deoxy-D-ribose in similar yields and selectivities to those achieved with the amino ester promoters (compare entries 3 and 9, or 6 and 12). Importantly, we also found that after 24 hours in an aqueous environment there was no observed erosion of the %ee of L-valine nitrile via polarimetry studies. Significantly, this is the first report of the promoter potential of these prebiotically important amino nitrile molecules.
Having demonstrated that amino nitriles can promote the deoxyribose-forming aldol reaction we turned our attention to completing the prebiotic synthesis of 2-deoxy-D-ribose from molecules available in interstellar space. This required overcoming the not insignificant challenge of efficient prebiotic formation of D-glyceraldehyde: the ultimate source of D-chirality in naturally occurring carbohydrates according to this proposal. Breslow has shown previously that D-glyceraldehyde could be formed in a small enantiomeric excess by the stoichiometric amino acid promoted aldol reaction of glycolaldehyde and formaldehyde,24,25 both of which are interstellar molecules39–42 and are also formed under prebiotic conditions in Sutherland's study.30 Weber has provided a more detailed investigation into the aldol reaction of glycolaldehyde and formaldehyde showing that a vast mixture of products can be formed under more rigorous conditions.43 Blackmond et al. have also shown how L-proline can be used to catalyze the formation of L-glyceraldehyde in an 8% ee from formaldehyde and glycolaldehyde, and that addition of an organic base to this reaction reversed and increased the ee to 13%.44 Breslow found that decreasing the pH of the reaction from neutral to pH 3.0–4.0 significantly increased the ee of D-glyceraldehyde.45
Using 1 mmol of glycolaldehyde dimer with 1 equivalent of formaldehyde and 20 mol% of L-valine nitrile in pH 7.0 buffer, we were able to form glyceraldehyde in situ after 24 hours (Scheme 1). Trapping with dinitrophenyl hydrazine using Breslow's procedure,24 gave trapped isolated glyceraldehyde in a 1% yield with an overall enantioselectivity of 6% in favour of the natural D-sugar; trapped starting materials accounted for the rest of the mass balance. This is in accordance with Breslow's findings, which recorded a 4.4% ee for D-glyceraldehyde using stoichiometric L-valine under similar conditions. When the reaction was run at pH 4.0, no glyceraldehyde was detected and the reaction returned only trapped starting materials. It is worth noting that the small initial enantiomeric excess of D-glyceraldehyde could, in principle, be readily amplified via concentration through water evaporation.24,46 As such, we report a two-step prebiotic synthesis to 2-deoxy-D-ribose employing amino esters or, for the first time, amino nitriles as promoters.
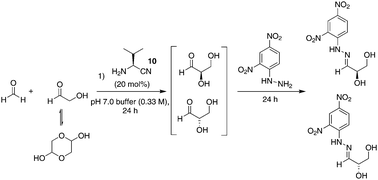 | ||
| Scheme 1 Formation of D-glyceraldehyde in pH 7.0 buffer, concentration of 0.33 M with respect to glycolaldehyde. | ||
With both steps of the prebiotic synthesis individually established we then investigated the synthesis of 2-deoxy-D-ribose in a one-pot reaction from the three interstellar building blocks, formaldehyde, acetaldehyde and glycolaldehyde, using amino ester L-7 and amino nitrile 10 as promoters. Both reactions were monitored by mass spectrometry and 1H NMR spectroscopy, and both were seen to be generating carbohydrate products after 24 hours, however, no 2-deoxy-D-ribose could be detected at this point – the reaction was therefore left for 7 days. Analysis showed a mixture of carbohydrate products, with the main components of both reactions being trapped starting materials. Using amino nitrile 10, 2-deoxy-D-ribose could not be identified amongst the carbohydrate products, which were trioses and tetroses 12–15 (Scheme 2). A control reaction between 10 and acetaldehyde identified a cyclic dimer (see ESI†) which we propose limits the formation of the desired pentose in the one pot process. However, we were delighted to observe that in the amino ester promoted reaction, both the mass spectrum of the crude reaction mixture and the mass spectrum after column chromatography contained a mass ion for 2-deoxypentose hydrazones (Fig. 1A). The HPLC trace of this fraction clearly showed a peak with the same retention time as 2-deoxy-D-ribose hydrazone (Fig. 1B). We could not detect any 2-deoxy-L-ribose in the fraction.36 Importantly, in the absence of amino ester or amino nitrile, only trapped starting reagents were isolated after 7 days, demonstrating the importance of the promoter.
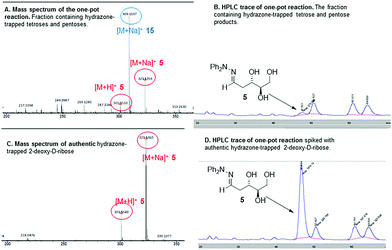 | ||
| Fig. 1 Mass spectra and HPLC data for the one-pot reaction, fractions containing tetrose and pentose products and authentic standards for comparison. | ||
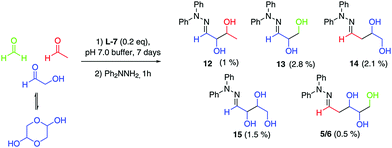 | ||
| Scheme 2 One-pot reaction using ‘Interstellar’ Building Blocks in pH 7.0 buffer at a concentration of 0.33 M with respect to glycolaldehyde. Yields are isolated yields after chromatography. | ||
We have shown for the first time that prebiotically important amino nitriles are capable of promoting the enantioselective aldol reaction of formaldehyde and glycolaldehyde to yield D-glyceraldehyde, and that subsequent reaction of the D-glyceraldehyde with acetaldehyde provides 2-deoxy-D-ribose – a simple two-step synthesis of a molecule of evolutionary importance from interstellar building blocks, we therefore recommend that amino nitriles should be considered as reagents and promoters by researchers exploring prebiotic reaction pathways. Further to this, we have demonstrated that it is possible to condense these steps into a one-pot synthesis of 2-deoxy-D-ribose, and other small sugars, by simple mixing of three achiral interstellar building blocks when using a chiral amino ester promoter. As such, we consider that this is an important step in understanding the possible prebiotic origins of 2-deoxy-D-ribose.
This research was supported by EPSRC DTG (York) programme (AMS) and the EU ERASMUS+ programme (NB). Reference spectroscopic and reaction data can be found at DOI: 10.15124/c62c6e39-7e18-4349-9441-4856badaf4d3.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. W. Powner, B. Gerland and J. D. Sutherland, Nature, 2009, 459, 239–242 CrossRef CAS PubMed.
- M. W. Powner, S.-L. Zheng and J. W. Szostak, J. Am. Chem. Soc., 2012, 134, 13889–13895 CrossRef CAS PubMed.
- C. Anastasi, M. A. Crowe, M. W. Powner and J. D. Sutherland, Angew. Chem., Int. Ed., 2006, 45, 6176–6179 CrossRef CAS PubMed.
- D. J. Ritson and J. D. Sutherland, J. Mol. Evol., 2014, 78, 245–250 CrossRef CAS PubMed.
- (a) L. Burroughs, M. E. Vale, J. A. R. Gilks, H. Forintos, C. J. Hayes and P. A. Clarke, Chem. Commun., 2010, 46, 4776–4778 RSC; (b) L. Burroughs, P. A. Clarke, H. Forintos, J. A. R. Gilks, C. J. Hayes, M. E. Vale, W. Wade and M. Zbytniewski, Org. Biomol. Chem., 2012, 10, 1565–1570 RSC.
- M. C. Lanning and S. S. Cohen, J. Biol. Chem., 1955, 216, 413–423 CAS.
- E. Racker, Nature, 1951, 167, 408–409 CrossRef CAS PubMed.
- L. E. Orgel, Origins Life Evol. Biospheres, 2003, 33, 211–218 CrossRef CAS.
- A. Lazcano and S. Miller, Cell, 1996, 85, 793–798 CrossRef CAS PubMed.
- A. Boutlerow, Comptes Rendus, 1861, 53, 145–147 Search PubMed.
- R. Breslow, Tetrahedron Lett., 1959, 1, 22–26 CrossRef.
- C. Appayee and R. Breslow, J. Am. Chem. Soc., 2013, 136, 3720–3723 CrossRef PubMed.
- P. Decker, H. Schweer and R. Pohlmann, J. Chromatogr., 1982, 244, 281–291 CrossRef CAS.
- A. Ricardo, M. A. Carrigan, A. N. Olcott and S. A. Benner, Science, 2004, 303, 196 CrossRef CAS PubMed.
- J. B. Lambert, S. A. Gurusamy-Thangavelu and M. Kuangbiao, Science, 2010, 327, 984–986 CrossRef CAS PubMed.
- J. B. Lambert, G. Lu, S. R. Singer and V. M. Kolb, J. Am. Chem. Soc., 2004, 126, 9611–9625 CrossRef CAS PubMed.
- (a) H.-J. Kim, A. Ricardo, H. I. Illangkoon, M. J. Kim, M. A. Carrigan, F. Frye and S. A. Benner, J. Am. Chem. Soc., 2011, 133, 9457–9468 CrossRef CAS PubMed; (b) S. A. Benner, H.-J. Kim and M. A. Carrigan, Acc. Chem. Res., 2012, 45, 2015–2034 CrossRef PubMed.
- S. Pizarello and A. L. Weber, Science, 2004, 303, 1151 CrossRef PubMed.
- A. Córdova, I. Ibrahem, J. Casas, H. Sundén, M. Engqvist and E. Reyes, Chem. – Eur. J., 2005, 11, 4772–4784 CrossRef PubMed.
- K. Kvenvolden, J. Lawless, K. Pering, E. Peterson, J. Flores, C. Ponnamperuma, I. R. Kaplan and C. Moore, Nature, 1970, 228, 923–926 CrossRef CAS PubMed.
- M. H. Engel and S. A. Macko, Nature, 1997, 389, 265–268 CrossRef CAS PubMed.
- Z. Martin, P. Modica, B. Zanda and L. L. D’Hendecourt, Meteorit. Planet. Sci., 2015, 50, 926–943 CrossRef.
- M. Klussmann, H. Iwamura, S. P. Mathew, D. H. Wells, Jr, U. Pandya, A. Armstrong and D. G. Blackmond, Nature, 2006, 441, 621–623 CrossRef CAS PubMed.
- R. Breslow and Z.-L. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5723–5725 CrossRef CAS PubMed.
- R. Breslow, V. Ramalingham and C. Appayee, Origins Life Evol. Biospheres, 2013, 43, 323–329 CrossRef PubMed.
- M. Tena-Solsona, J. Nanda, S. Díaz-Oltra, A. Chotera, G. Ashkenasy and B. Escuder, Chem. – Eur. J., 2016, 22, 6687–6694 CrossRef CAS PubMed.
- A. L. Weber and S. Pizzarello, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12713–12717 CrossRef CAS PubMed.
- J. Kofoed, J.-L. Reymond and T. Darbre, Org. Biomol. Chem., 2005, 3, 1850–1855 CAS.
- J. Kofoed, M. Machuqueiro, J.-L. Reymond and T. Darbre, Chem. Commun., 2004, 1540–1541 RSC.
- B. H. Patel, C. Percivalle, D. J. Ritson, C. D. Duffy and J. D. Sutherland, Nat. Chem., 2015, 7, 301–307 CrossRef CAS PubMed.
- M. de Marcellus, C. Meinert, I. Myrgorodska, L. Nahon, T. Buhse, L. Le Sergeant d’Hendecourt and U. J. Meierhenrich, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 965–970 CrossRef PubMed.
- C. A. Gottlieb, in Molecules in the Galactic Environment, ed. M. A. Gordon and L. E. Snyder, Wiley-Interscience, New York, 1973, pp. 181–186 Search PubMed.
- S. Pizarello and A. L. Weber, Origins Life Evol. Biospheres, 2010, 40, 3–10 CrossRef PubMed.
- R. Breslow and C. Appayee, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4184–4187 CrossRef CAS PubMed.
- J. Oro and A. C. Cox, Fed. Proc., 1962, 21, 80 Search PubMed.
- See ESI†.
- T. Kawasaki, N. Takamatsu, S. Aiba and Y. Tokunaga, Chem. Commun., 2015, 51, 14377–14380 RSC.
- S. Miyagawa, K. Yoshimura, Y. Yamazaki, N. Takamatsu, T. Kuraishi, S. Aiba, Y. Tokunaga and T. Kawasaki, Angew. Chem., Int. Ed., 2017, 56, 1055–1058 CrossRef CAS PubMed.
- L. E. Snyder, D. Buhl, B. Zuckerman and P. Palmer, Phys. Rev. Lett., 1969, 22, 679 CrossRef CAS.
- J. M. Hollis, F. J. Lovas and P. R. Jewell, Astrophys. J., 2000, 540, L107–L110 CrossRef CAS.
- J. K. Jørgensen, C. Favre, S. E. Bisschop, T. L. Bourke, E. F. van Dishoeck and M. Schmalzl, Astrophys. J., Lett., 2012, 757, L4 CrossRef.
- C. Meinert, Science, 2016, 352, 208–212 CrossRef CAS PubMed.
- A. L. Weber, Origins Life Evol. Biospheres, 2001, 31B, 71–86 CrossRef.
- J. E. Hein and D. G. Blackmond, Acc. Chem. Res., 2012, 45, 2045–2054 CrossRef CAS PubMed.
- R. Breslow and V. Ramalingam, Origins Life Evol. Biospheres, 2013, 43, 323–329 CrossRef PubMed.
- R. Breslow, Tetrahedron Lett., 2011, 52, 2028–2032 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, synthesis of authentic standards and copies of spectroscopic data. See DOI: 10.1039/c7cc06083a |
| This journal is © The Royal Society of Chemistry 2017 |