Open Access Article
Open Access ArticleA luminescent cationic metal–organic framework featuring [Cu–pyrazolate]3 units for volatile organic compound sensing†
Charlie E.
Kivi
and
Datong
Song
*
Davenport Chemical Research Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada M5S 3H6. E-mail: dsong@chem.utoronto.ca
First published on 6th October 2016
Abstract
A new metal–organic framework (1) constructed from 4-(4-(3,5-dimethyl-1H-pyrazol-4-yl)phenyl)pyridine (HL) and CuBr shows potential as a luminescent sensor for volatile organic compounds.
Metal–organic frameworks (MOFs) have garnered a lot of recent attention due to their many applications.1 Of particular interest to us is the ability of MOFs to perform as luminescent chemical sensors for volatile organic compounds (VOCs).2,3 While most current industrial VOC sensing is done via photo ionization detection (PID)4,5 or other solid state detection methods,6 sensing using supramolecular structures has been known for some time.7 Low energy, reliable VOC sensors with high sensitivity are desirable industrially to allow for the rapid detection of hazardous compounds (e.g., solvent vapours). While there are many recent examples, most known luminescent MOF sensors for VOCs rely on ligand-based8,9 or lanthanide-based10,11 emissions.2,12,13 In contrast, luminescence involving the transition metal centres (e.g., metal–ligand charge transfer)14,15 remains relatively underexplored in MOFs. Some known examples include Lin's RuII–bipyridine MOF16 and Mączka's CrIII MOF.17
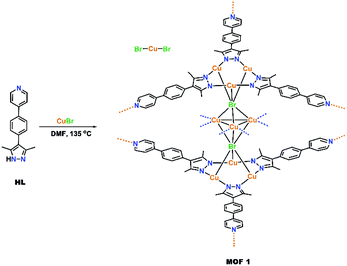
The trinuclear CuI–pyrazolate (Cu3Pz3) chromophore caught our attention due to its proven sensing ability in homogeneous solution18 and the active role of the CuI centres in luminescence.19–21 Simple luminescent CuI3Pz3 complexes consisting of prisms,21,22 cages23–25 and oligomers26 are known. To date, there are only six reported MOF types that utilize the CuI3Pz3 building blocks,27–34 among which those with the pyridyl (Py) functional group on the ligand are most relevant to sensing.31–34 However, only thermochromism31,33 and a “chemopalette effect” (i.e., doping the MOF with different coordinating solvents during synthesis)34 were reported for the known Py-functionalized CuI3Pz3-containing MOFs. All the known CuI3Pz3-containing MOFs have charge neutral skeletons with no reported role of the species in the pores impacting MOF luminescent behaviour.27–34 Herein we report an MOF synthesized from a new ligand, 4-(4-(3,5-dimethyl-1H-pyrazol-4-yl)phenyl)pyridine (HL), which features the CuI3Pz3 chromophore and a cationic skeleton with [CuIBr2]− counterions in the pores. Preliminary experiments demonstrate the sensing ability of this material towards VOCs.
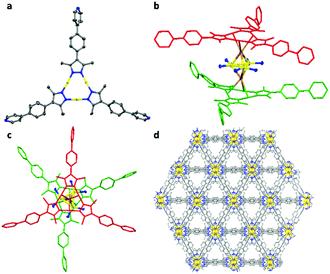
Yellow crystals of [Cu9L6Br2][CuBr2] (1) can be obtained in moderate yields by heating CuBr and HL in dry, degassed DMF under an N2 atmosphere at 135 °C for four days (Scheme 1). The cationic charge of the framework is balanced by [CuBr2]− anions residing in the pores. Two building blocks can be found in the skeleton of 1: (a) trigonal [Cu3L3] units, similar to the known [Cu3Pz3] compounds and (b) trigonal bipyramidal shaped [Cu3Br2]+ clusters with the two bromides occupying the apical positions (Fig. 1a and b). The repeating unit of the cationic skeleton of 1 can then be described as a trigonal bipyramidal shaped [CuI3Br2]+ cluster sandwiched in between two staggered trigonal [CuI3L3] layers, with each apical bromide of the [CuI3Br2]+ cluster interacting with all three CuI centres of one [CuI3L3] unit (Fig. 1b and c). Each repeating unit in Fig. 1b is linked to six adjacent units through the coordination of the pyridine nitrogen donor from one unit (shown in blue in Fig. 1b) to the CuI centre in the middle of the other unit, resulting in the formation of a 3D framework (see the ESI† for further descriptions). There are trigonal prismatic channels along the a + c direction of the crystal lattice, where the disordered counterions and solvent molecules reside (Fig. 1d).
MOF 1 shows broadband excitation and emission spectra in the solid state with excitation and emission maxima at 469 and 560 nm, respectively (Fig. S7†) and a quantum yield of 8.6%. Treatment of 1 with water vapour to eliminate any residual channel organic solvents results in a decrease of quantum yield to 1.8%. The water-treated sample is used as the baseline for VOC sensing experiments. Representatives of each common solvent type were compared (Fig. 2). Ethyl acetate, benzene and pentane all show notable turn-on behaviour with a greater than 60% increase of luminescence intensity versus the water baseline indicating that the MOF may be a potential sensor for esters, alkanes and aromatics. Acetone shows a slight increase (20%), while acetonitrile, tetrahydrofuran (THF)‡ and methanol all have negligible impact on luminescence intensity (Fig. S19†). Diethyl ether and chloroform both cause notable turn-off with a luminescence intensity decrease of more than 25%. Notably, ethyl acetate vapour causes the most significant luminescence intensity enhancement and the quantum yield of the ethyl acetate-treated MOF is 24.3%. Such a high quantum yield allows us to measure the luminescence lifetime accurately. Multiple lifetimes were observed (Tables S4 and S5†) with the longest being 2.6 μs, which is comparable to other MOFs containing CuI3Pz3 units and is indicative of metal-based phosphorescence.32–34 Lastly, some solvochromism is observed but the overall effect is minimal, i.e., all luminescent maxima are within the range of 555 to 585 nm (Table S2†).
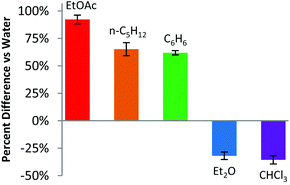 | ||
| Fig. 2 Abridged VOC sensing results.§ An excitation wavelength of 469 nm was used and the luminescence response was measured at the emission maximum (Table S2†) for each solvent treated sample. | ||
In order to probe whether the key luminescent changes are the results of MOF structural changes or surface effects, a structure–activity study was conducted. Since ethyl acetate yielded the highest luminescent enhancement, the structures of MOF samples treated with ethyl acetate and water vapour were examined via PXRD experiments. Treatment of as-synthesized 1 with water vapour shows a significant alteration of the PXRD pattern indicative of a structural change. The subsequent treatment with ethyl acetate vapour results in the restoration of the PXRD pattern of as-synthesized 1. Sequential exposures of as-synthesized 1 to water and ethyl acetate vapours yield reversible structural changes as evidenced by the cycling of PXRD patterns (Fig. S10†). All other solvents (except for ether) cause a similar structural change as ethyl acetate (Fig. S11–S18†).
Based on the studies of similar compounds in the literature,32 the luminescence of 1 likely originates from the metal-to-metal charge transfer 3[MMCT], metal-to-ligand charge transfer 3[MLCT], and halide-to-ligand charge transfer 3[XLCT] excited states. The 3[MMCT] excited state is likely the main contributor, evidenced by the broad and featureless emission spectrum of 1. All three types of excited states are well-known contributors for related luminescent Cu(I) complexes.15,20,32,35 Coordinating solvents (such as water, acetonitrile, alcohols, and THF) are known to quench the luminescence of Cu(I) compounds via exciplex formation.36,37 Presumably, the more substantial turn-on caused by water-immiscible solvents (i.e., EtOAc, pentane, benzene) is due to their non-coordinating nature and ability to displace the quencher water from the pores. Conversely, although the water-miscible solvents (i.e., acetonitrile, THF, acetone, and methanol) may also displace water molecules in the pore as shown by the PXRD experiments, which should cause luminescence enhancement, these solvents can quench luminescence via exciplex formation due to their coordinating nature. Therefore, most of these solvents cause no significant luminescence intensity change overall. Only the less coordinating acetone gives a 25% turn-on compared to water-treated 1.
Since chloroform is able to displace water and halocarbons are well known to quench the transition metal-based luminescence through electron transfer,38 the interactions between chloroform and the luminescent sites within the pores likely account for the observed luminescence intensity decrease. On the other hand, ether causes no structural change to the water-treated 1 (Fig. S18†), implying that the luminescence quenching caused by ether is a surface effect.
In summary, we have prepared a new luminescent Cu(I)–pyrazolate MOF 1, featuring a [Cu3Pz3] triangle and Cu(I) cluster building units and a cationic skeleton. The exposure of water-treated 1 to the vapours of water-immiscible VOCs causes luminescence signal modulations, which can potentially be used for the detection of these VOCs. Preliminary studies show that most VOCs cause structural changes to the water-treated 1, except for ether. Further investigations into the origin of the luminescence signal modulation and isoreticular MOFs of the functionalized HL ligand for selective VOC sensing are being carried out in our laboratory.
Acknowledgements
We thank NSERC of Canada for funding. We also acknowledge the Canadian Foundation for Innovation Project #19119, and the Ontario Research Fund for funding the CSICOMP NMR lab at the University of Toronto enabling the purchase of several new spectrometers. We thank Mr. Andrew H. Proppe for his assistance on luminescence lifetime and quantum yield measurements.Notes and references
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC and references therein.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- P. C. Hsi (Rae Systems, Inc.), WO1994027141A1, 1994 Search PubMed; P. C. Hsi (Rae Systems, Inc.), US Pat, 5393979, 1995 Search PubMed; P. C. Hsi (Rae Systems, Inc.), US Pat, 5561344A, 1996 Search PubMed.
- J. D. Coy, P. L. Bigelow, R. M. Buchan, J. D. Tessari and J. O. Parnell, AIHAJ, 2000, 61, 268–274 CAS.
- Solid state gas sensing, ed. E. Comini, G. Faglia, and G. Sberveglieri, Springer, New York, N.Y., 2009 Search PubMed.
- L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS PubMed.
- M. M. Wanderley, C. Wang, C.-D. Wu and W. Lin, J. Am. Chem. Soc., 2012, 134, 9050–9053 CrossRef CAS PubMed.
- M. Zhang, G. Feng, Z. Song, Y.-P. Zhou, H.-Y. Chao, D. Yuan, T. T. Y. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 7241–7244 CrossRef CAS PubMed.
- Y. Li, S. Zhang and D. Song, Angew. Chem., Int. Ed., 2013, 52, 710–713 CrossRef CAS PubMed.
- C. Zhan, S. Ou, C. Zou, M. Zhao and C.-D. Wu, Anal. Chem., 2014, 86, 6648–6653 CrossRef CAS PubMed.
- D. Zhao, Y. Cui, Y. Yang and G. Qian, CrystEngComm, 2016, 18, 3746–3759 RSC.
- L. Zhang, Z. Kang, X. Xin and D. Sun, CrystEngComm, 2016, 18, 193–206 RSC.
- V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589–7728 CrossRef CAS PubMed.
- A. Vogler and H. Kunkely, in Transition Metal and Rare Earth Compounds, ed. H. Yersin, Springer, Berlin, Heidelberg, 2001, pp. 143–182 Search PubMed.
- C. A. Kent, D. Liu, T. J. Meyer and W. Lin, J. Am. Chem. Soc., 2012, 134, 3991–3994 CrossRef CAS PubMed.
- M. Mączka, B. Bondzior, P. Dereń, A. Sieradzki, J. Trzmiel, A. Pietraszko and J. Hanuza, Dalton Trans., 2015, 44, 6871–6879 RSC.
- H. V. R. Dias, H. V. K. Diyabalanage, M. G. Eldabaja, O. Elbjeirami, M. A. Rawashdeh-Omary and M. A. Omary, J. Am. Chem. Soc., 2005, 127, 7489–7501 CrossRef CAS PubMed.
- M. A. Omary, M. A. Rawashdeh-Omary, M. W. A. Gonser, O. Elbjeirami, T. Grimes, T. R. Cundari, H. V. K. Diyabalanage, C. S. P. Gamage and H. V. R. Dias, Inorg. Chem., 2005, 44, 8200–8210 CrossRef CAS PubMed.
- B. Hu, G. Gahungu and J. Zhang, J. Phys. Chem. A, 2007, 111, 4965–4973 CrossRef CAS PubMed.
- G.-F. Gao, M. Li, S.-Z. Zhan, Z. Lv, G. Chen and D. Li, Chem. – Eur. J., 2011, 17, 4113–4117 CrossRef CAS PubMed.
- M. Veronelli, S. Dechert, S. Demeshko and F. Meyer, Inorg. Chem., 2015, 54, 6917–6927 CrossRef CAS PubMed.
- P.-C. Duan, Z.-Y. Wang, J.-H. Chen, G. Yang and R. G. Raptis, Dalton Trans., 2013, 42, 14951–14954 RSC.
- M. Grzywa, B. Bredenkötter, D. Denysenko, S. Spirkl, W. Nitek and D. Volkmer, Z. Anorg. Allg. Chem., 2013, 639, 1461–1471 CrossRef CAS.
- T. Jozak, Y. Sun, Y. Schmitt, S. Lebedkin, M. Kappes, M. Gerhards and W. R. Thiel, Chem. – Eur. J., 2011, 17, 3384–3389 CrossRef CAS PubMed.
- J.-H. Wang, M. Li, J. Zheng, X.-C. Huang and D. Li, Chem. Commun., 2014, 50, 9115–9118 RSC.
- J. He, Y.-G. Yin, T. Wu, D. Li and X.-C. Huang, Chem. Commun., 2006, 2845–2847 RSC.
- J.-P. Zhang and S. Kitagawa, J. Am. Chem. Soc., 2008, 130, 907–917 CrossRef CAS PubMed.
- Z. Wei, D. Yuan, X. Zhao, D. Sun and H.-C. Zhou, Sci. China: Chem., 2013, 56, 418–422 CrossRef CAS.
- J.-H. Wang, M. Li and D. Li, Chem. – Eur. J., 2014, 20, 12004–12008 CrossRef CAS PubMed.
- J.-X. Zhang, J. He, Y.-G. Yin, M.-H. Hu, D. Li and X.-C. Huang, Inorg. Chem., 2008, 47, 3471–3473 CrossRef CAS PubMed.
- L. Hou, W.-J. Shi, Y.-Y. Wang, H.-H. Wang, L. Cui, P.-X. Chen and Q.-Z. Shi, Inorg. Chem., 2011, 50, 261–270 CrossRef CAS PubMed.
- S.-Z. Zhan, M. Li, X.-P. Zhou, J.-H. Wang, J.-R. Yang and D. Li, Chem. Commun., 2011, 47, 12441–12443 RSC.
- S.-Z. Zhan, M. Li, S. W. Ng and D. Li, Chem. – Eur. J., 2013, 19, 10217–10225 CrossRef CAS PubMed.
- P. C. Ford, E. Cariati and J. Bourassa, Chem. Rev., 1999, 99, 3625–3648 CrossRef CAS PubMed.
- D. R. McMillin, J. R. Kirchhoff and K. V. Goodwin, Coord. Chem. Rev., 1985, 64, 83–92 CrossRef CAS.
- J. V. Lockard, S. Kabehie, J. I. Zink, G. Smolentsev, A. Soldatov and L. X. Chen, J. Phys. Chem. B, 2010, 114, 14521–14527 CrossRef CAS PubMed.
- H.-K. Yip, H.-M. Lin, K.-K. Cheung, C.-M. Che and Y. Wang, Inorg. Chem., 1994, 33, 1644–1651 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. CCDC 1494011. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt03582e |
| ‡ In order to test the role of residual water in the solvent, the performance of the MOF was examined with wet (reagent grade THF from a commercial bottle) versus dry (distilled over sodium/benzophenone ketyl and stored over 3 Å molecular sieves in a glovebox) THF was compared (Fig. S20†). No significant difference was observed. |
| § Full figure available in ESI (Fig. S19†). |
| This journal is © The Royal Society of Chemistry 2016 |