 Open Access Article
Open Access ArticleControlled formation of ordered coordination polymeric networks using silsesquioxane building blocks†
Subhabrata
Banerjee
,
Sho
Kataoka
 *,
Toshikazu
Takahashi
,
Yoshihiro
Kamimura
,
Kunio
Suzuki
,
Kazuhiko
Sato
and
Akira
Endo
*
*,
Toshikazu
Takahashi
,
Yoshihiro
Kamimura
,
Kunio
Suzuki
,
Kazuhiko
Sato
and
Akira
Endo
*
National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: s-kataoka@aist.go.jp; endo-akira@aist.go.jp
First published on 22nd August 2016
Abstract
In this report, we synthesized ordered coordination polymers using polyhedral oligomeric silsesquioxanes (POSS) as a building block. A POSS with eight carboxylic terminals was coordinated with copper ions at various temperatures, forming polymeric networks. This novel coordination polymer has a long-range ordered structure.
Polyhedral oligomeric silsesquioxanes (POSS) have a unique cage-like structure with the general formula RSiO1.5 and have been used in a wide range of applications as hybrid materials.1,2 In recent years, several efforts have been made to exploit POSS as a building block for synthesising ordered materials, and some schemes have been proposed.3–14 As one of the most accepted schemes, POSS were first tethered with chemical moieties and then self-assembled/crosslinked to form higher-order structures via intermolecular interactions. For example, Shimojima et al. synthesised porous materials by modifying POSS terminals with an alkyl group3 or dimethylsilanol groups.7 Chu et al. prepared POSS with 8 acetamido-pyridine terminals and made an electroluminescent material.5 Huang et al. modified tetrakis(4-azidophenyl)methane terminals with hydrophilic or hydrophobic POSS cages and formed self-assembling materials.15 Recently, Janeta et al. synthesized several imino functionalized POSS and obtained well-defined crystals.13 We also employed a POSS with propylammonium groups as an interlayer for the fabrication of crystalline layered perovskite materials.6
Coordinate bonds are known to form extended networks with well-defined geometries and have been widely used for metal–organic coordination polymers. In these coordination polymers, organic ligands are typically coordinated to transition metal centres.16,17 While many organic ligands have been designed to obtain coordination polymers with characteristic long-range ordered structures, POSS can also be ligands of coordination polymers. Du et al. synthesized a POSS with 8-hydroxyquinoline terminals to conjugate them with Zn.18 Köytepe et al. synthesized terpyridine-functionalized POSS and formed coordination networks with Co and Cu.19 Xu et al. modified 8 aminopropyl terminals of POSS with 4-carboxybenzaldehyde and reacted them with Tb.20 However, despite the creation of coordinate bonds with metal centres, no long-range ordered structure has been confirmed for their coordination polymers in these reports. One possible reason for this scarcity is the complexity of conjugating the 8 terminal groups of POSS equally to the metal centres in a desired manner.
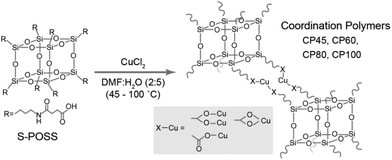
In this study, we control the reaction temperature of coordinate bond formation and fabricate ordered polymeric networks using a POSS scaffold. First, an aminopropyl terminal POSS was synthesized using a mixture of (3-aminopropyl)triethoxysilane, hydrochloric acid, and methanol according to previous literature reports.21,22 The prepared aminopropyl terminal POSS was further treated with succinic anhydride to obtain a POSS with eight carboxylic terminals (hereafter referred to as S-POSS).22 The prepared S-POSS was used as a ligand for making a network with metal centres. Mixtures of 80 mg of S-POSS in 4 mL DMF and 62.2 mg of CuCl2 in 10 mL water were heated at several temperatures (45, 60, 80, and 100 °C) while stirring overnight (approximately 16 h). Hereafter, each coordination polymer is referred to as CP45 (yield; 70.1 mg), CP60 (43.5 mg), CP80 (43.9 mg), and CP100 (55.1 mg), respectively. After the reaction, the precipitate was filtered and washed with methanol (Scheme 1).
The obtained coordination polymers and S-POSS were observed using a field emission scanning electron microscope (FE-SEM). FE-SEM images are shown in Fig. 1. In the image of S-POSS, needle-like crystals grow radially from the centre. CP45 also contains needle-like crystals, which are apparently similar to the S-POSS image. This indicates that CP45 has a crystal structure similar to S-POSS and retains the nature of S-POSS. These coordination polymers exhibit monodispersed plate-like microstructures with an increase in the reaction temperature. Both CP60 and CP80 exhibit a thick plate-like shape. At temperatures above 80 °C, CP100 exhibits a plate-like shape similar to CP60 and CP80, but has smaller and more randomly-formed particles than CP60 and CP80. These changes in shape are possibly related to the degree of coordinate bond formation.
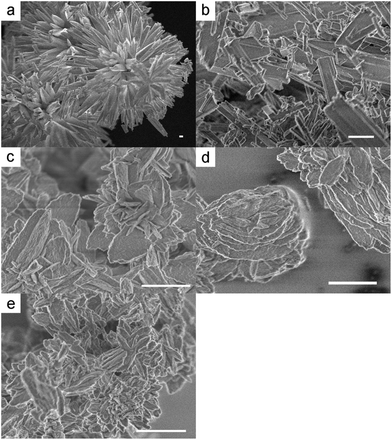 | ||
| Fig. 1 Field emission scanning electron microscopy (FE-SEM) images of S-POSS and the obtained coordination polymers: (a) S-POSS, (b) CP45, (c) CP60, (d) CP80, and (e) CP100 (scale bar: 1 μm). | ||
Powder X-ray diffraction (PXRD) patterns of the obtained coordination polymers were analysed for the investigation of their crystalline nature (Fig. 2). The PXRD pattern of S-POSS exhibits sharp peaks ranging from 18° to 25°, which is presumably related to the POSS cages (approximately 5 Å).2 The pattern of CP45 shows peaks similar to that of S-POSS as well as peaks appearing at angles lower than 15°, especially at 7.7° (d = 11.5 Å). For CP60 and CP80, the peaks from 3° to 15° are predominant while those in the range 18°–25° are attenuated except for that at 22.3° (d = 3.98 Å). In particular, a sharp peak at 3.8° corresponds to a d-spacing of 23.3 Å, nearly equivalent to the size of S-POSS (24 Å, see also Fig. S1 in the ESI†).
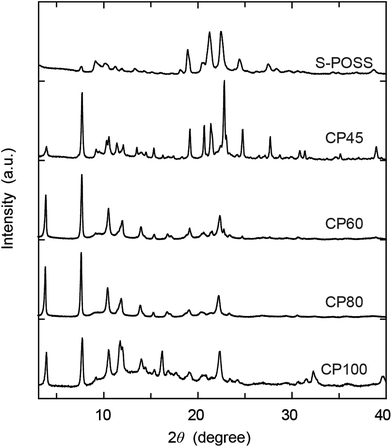 | ||
| Fig. 2 Powder X-ray diffraction (PXRD) patterns of the S-POSS ligand and coordination polymers prepared at various temperatures. | ||
Therefore, the results clearly suggest the formation of long-range ordered networks of S-POSS. The pattern of CP100 is almost the same as those of CP60 and CP80 except for the noticeable diffused bands observed in the range 10°–25°, ascribed to amorphous materials. Although we have not been able to thoroughly refine these patterns, we speculate that the crystalline structures of CP60 and CP80 are orthorhombic (see Fig. S2 in the ESI†).
Fourier transform infrared (FTIR) spectra were measured for further understanding the coordinate bond formation. Major peaks and their assignments are presented in Fig. 3.10,23–25 The spectra of coordination polymers show some characteristic differences from that of S-POSS through the coordination reaction to Cu2+. The bands of the carboxyl group (1197 and 1695 cm−1) that clearly appear in the S-POSS spectrum are attenuated for CP45 and are not present for CP60, CP80, and CP100. Meanwhile, a band at 1588 cm−1 newly appears for CP45 and is intensified for CP60, CP80, and CP100. Furthermore, the peak at 1427 cm−1 is slightly shifted to 1438 cm−1 with an increase in the reaction temperature. These peak shifts reveal that the carboxyl terminals of the S-POSS were coordinated to Cu2+ and that the extent of coordination gradually increased with an increase in the reaction temperature.20,26 In addition, the S-POSS itself is intact in the coordination polymers from the solid-state NMR results (see Fig. S3 in the ESI†). Accordingly, the Si–O–Si stretching peak (1103 cm−1) and the amide bands (1544 and 1643 cm−1) remain unchanged between S-POSS and all the coordination polymers.
As for the carboxylate groups that newly appeared for CP60, CP80, and CP100 (1588 and 1438 cm−1), the gap between the asymmetric and symmetric bands, Δv is 150 cm−1. These results infer that the carboxyl groups of S-POSS ligands form a bridging bidentate coordination to Cu atoms.23,24,27–29
Furthermore, inductively coupled plasma (ICP) data were collected for all coordination polymers to obtain the fraction of the Cu and Si species. The Cu and Si weight fractions obtained from the ICP data are presented in Table 1. Based on these numbers, the Si/Cu ratio and X were calculated. The Cu fraction increases with an increase in the reaction temperature, while the Si/Cu ratio markedly varies with the reaction temperature. The degree of the network formation between the carboxylate terminals and Cu2+ is sensitive to the reaction temperature, which is in good agreement with the previous results. For the CP60 and CP80 cases, the Si/Cu ratio is approximately 2. This indicates that two carboxylate ligands are bound to each copper on average with the formula [Si8O12(C7H11NO3)8][Cu2+]4. From the FTIR results, the carboxylate ligands form a bridging bidentate coordination to Cu2+ cations. Accordingly, four carboxylate terminals on POSS appear to be coordinated to two copper metals and form paddle-wheel units.28 This is also supported by the UV-vis spectra (see Fig. S4 in the ESI†).30 For CP45 at Si/Cu = 10.6, the coordinate bond formation is incomplete whereas the coordination polymer has partially random networks in the structure in the case of CP100 at Si/Cu = 0.867.
| Fraction (wt%) | ||||
|---|---|---|---|---|
| Materials | Cu | Si | Si/Cu ratio | X |
| a X: with the chemical formula [Si8O12(C7H11NO3)8][Cu2+]X. | ||||
| CP45 | 2.45 | 11.5 | 10.6 | 0.75 |
| CP60 | 10.1 | 9.89 | 2.19 | 3.65 |
| CP80 | 12.2 | 11.1 | 2.05 | 3.90 |
| CP100 | 21.3 | 8.17 | 0.867 | 9.22 |
Thermogravimetric analysis (TGA) of S-POSS and all coordination polymers was conducted to confirm their thermal stabilities (Fig. 4). CP45 exhibited a step-wise mass loss, which is quite different from that of S-POSS and the other coordination polymers. This indicates that it is a mixture of components and contains an insufficient coordination network formation. CP60, CP80, and CP100 were thermally decomposed around 230 °C. Importantly, the weight fraction at 500 °C was approximately 31.3% for CP60, 33.1% for CP80, and 41.5% for CP100. This trend is consistent with the ICP data.
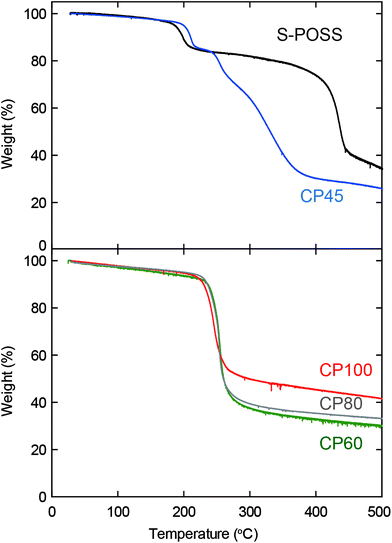 | ||
| Fig. 4 Thermogravimetric analysis (TGA) diagram of S-POSS and coordination polymers (CP45, CP60, CP80, and CP100). | ||
From the results of FE-SEM, PXRD, FTIR, ICP, and TGA when the coordinate bond formation is incomplete at a low reaction temperature (45 °C), CP45 retains the nature of S-POSS. At a high reaction temperature (100 °C), CP100 contains the random polymeric network. The desired crystalline polymeric network can be obtained only at suitable temperatures between 60 °C and 80 °C. This temperature dependence is interpreted in the following way. When water was added to S-POSS dissolved in DMF in the absence of Cu2+, S-POSS was partially precipitated within 30 min in our experimental procedure because of the low solubility of S-POSS in water. Accordingly, when S-POSS in DMF and Cu2+ in water were mixed, two competing reactions were involved: the precipitation through the removal of DMF solvated around S-POSS and the coordinate bond formation between Cu2+ and S-POSS. Therefore, a precise reaction temperature control is required for making crystalline coordination polymers.
In addition, we conducted nitrogen sorption measurements at 77 K for all coordination polymers (see Fig. S5 in the ESI†). The results indicated that the polymers adsorbed a minimal amount of nitrogen molecules. Although the obtained coordination polymers have a long-range ordered network similar to typical coordination polymers with organic ligands, they are almost non-porous. We speculate that this is caused by the strong interaction between the amide groups and the length of the S-POSS side chains. The side chain having a C7H11NO3 chemical formula (approximately 10 Å) is relatively long compared to the POSS cage (approximately 5 Å).2 Therefore, the S-POSS side chains are close to each other, which results in their non-porous structure (see Fig. S1 in the ESI†).
In conclusion, we developed a synthetic method for coordination polymers with a long-range ordered structure using POSS and copper chloride. We conducted a detailed study of the formation of coordination networks between S-POSS ligands at various reaction temperatures. We also confirmed the structural and chemical identity of the prepared materials through FE-SEM, PXRD, FTIR, and ICP analyses. Finally, we demonstrated that a POSS with 8 terminal groups can be employed as a ligand for coordination polymers.
This work was fully supported by the “Development of Innovative Catalytic Processes for Organosilicon Functional Materials” project (PL: K. S.) from the New Energy and Industrial Technology Development Organization (NEDO). We also thank Ms Rie Satou and Mr Wako Kaburagi (AIST) for their assistance with ICP measurements and sample preparation.
Notes and references
- R. M. Laine, J. Mater. Chem., 2005, 15, 3725–3744 RSC.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- A. Shimojima, R. Goto, N. Atsumi and K. Kuroda, Chem. – Eur. J., 2008, 14, 8500–8506 CrossRef CAS PubMed.
- D. M. L. Goodgame, S. Kealey, P. D. Lickiss and A. J. P. White, J. Mol. Struct., 2008, 890, 232–239 CrossRef CAS.
- Y. L. Chu, C. C. Cheng, Y. P. Chen, Y. C. Yen and F. C. Chang, J. Mater. Chem., 2012, 22, 9285–9292 RSC.
- S. Kataoka, S. Banerjee, A. Kawai, Y. Kamimura, J.-C. Choi, T. Kodaira, K. Sato and A. Endo, J. Am. Chem. Soc., 2015, 137, 4158–4163 CrossRef CAS PubMed.
- N. Sato, Y. Kuroda, T. Abe, H. Wada, A. Shimojima and K. Kuroda, Chem. Commun., 2015, 51, 11034–11037 RSC.
- M.-Y. Zhang, K.-H. Gu, Y. Zhou, S. Zhou, X.-H. Fan and Z. Shen, Chem. Commun., 2016, 52, 3923–3926 RSC.
- A. Boullanger, G. Gracy, N. Bibent, S. Devautour-Vinot, S. Clement and A. Mehdi, Eur. J. Inorg. Chem., 2012, 143–150 CrossRef CAS.
- X. Ge, L. Dong, L. Sun, Z. Song, R. Wei, L. Shi and H. Chen, Nanoscale, 2015, 7, 7206–7215 RSC.
- G. Chen, Y. Zhou, X. Wang, J. Li, S. Xue, Y. Liu, Q. Wang and J. Wang, Sci. Rep., 2015, 5, 11236 CrossRef PubMed.
- V. Ervithayasuporn, K. Kwanplod, J. Boonmak, S. Youngme and P. Sangtrirutnugul, J. Catal., 2015, 332, 62–69 CrossRef CAS.
- M. Janeta, L. John, J. Ejfler, T. Lis and S. Szafert, Dalton Trans., 2016, 45, 12312–12321 RSC.
- S. Chimjarn, R. Kunthom, P. Chancharone, R. Sodkhomkhum, P. Sangtrirutnugul and V. Ervithayasuporn, Dalton Trans., 2015, 44, 916–919 RSC.
- M. J. Huang, C. H. Hsu, J. Wang, S. Mei, X. H. Dong, Y. W. Li, M. X. Li, H. Liu, W. Zhang, T. Z. Aida, W. B. Zhang, K. Yue and S. Z. D. Cheng, Science, 2015, 348, 424–428 CrossRef CAS PubMed.
- N. N. Adarsh and P. Dastidar, Chem. Soc. Rev., 2012, 41, 3039–3060 RSC.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- F. F. Du, H. Wang, Y. Y. Bao, B. Liu, H. T. Zheng and R. K. Bai, J. Mater. Chem., 2011, 21, 10859–10864 RSC.
- S. Köytepe, M. H. Demirel, A. Gultek and T. Seckin, Polym. Int., 2014, 63, 778–787 CrossRef.
- Q. Q. Xu, Z. Q. Li, M. Chen and H. R. Li, CrystEngComm, 2016, 18, 177–182 RSC.
- F. J. Feher and K. D. Wyndham, Chem. Commun., 1998, 323–324 RSC.
- K. Tanaka, K. Inafuku, S. Adachi and Y. Chujo, Macromolecules, 2009, 42, 3489–3492 CrossRef CAS.
- Y. K. Seo, G. Hundal, I. T. Jang, Y. K. Hwang, C. H. Jun and J. S. Chang, Microporous Mesoporous Mater., 2009, 119, 331–337 CrossRef CAS.
- K. Tan, N. Nijem, P. Canepa, Q. Gong, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2012, 24, 3153–3167 CrossRef CAS.
- J. Wang, H.-Z. Geng, Z.-J. Luo, S. Zhang, J. Zhang, J. Liu, H.-J. Yang, S. Ma, B. Sun, Y. Wang, S.-X. Da and Y.-Q. Fu, RSC Adv., 2015, 5, 105393–105399 RSC.
- K. Hirai, B. Yeom, S.-H. Chang, H. Chi, J. F. Mansfield, B. Lee, S. Lee, C. Uher and N. A. Kotov, Angew. Chem., Int. Ed., 2015, 54, 8966–8970 CrossRef CAS PubMed.
- K. S. Finnie, J. R. Bartlett and J. L. Woolfrey, Langmuir, 1998, 14, 2744–2749 CrossRef CAS.
- E. Ahvenniemi and M. Karppinen, Chem. Commun., 2016, 52, 1139–1142 RSC.
- K. B. Klepper, O. Nilsen, S. Francis and H. Fjellvag, Dalton Trans., 2014, 43, 3492–3500 RSC.
- K. Wojciechowski, A. Bitner, G. Bernardinelli and M. Brynda, Dalton Trans., 2009, 1114–1122 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and TGA data. See DOI: 10.1039/c6dt02868c |
| This journal is © The Royal Society of Chemistry 2016 |

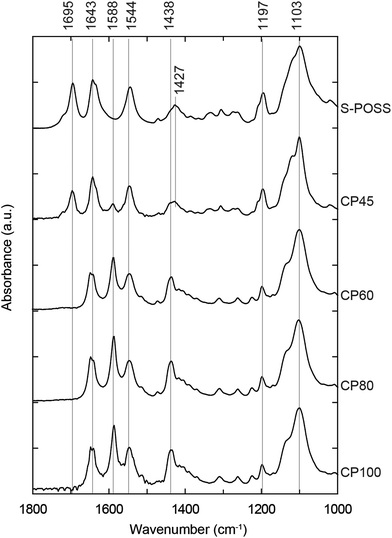
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the carboxyl group (1197 and 1695 cm−1); the asymmetric stretching band of the carboxylate group (1427 cm−1); and the N–H bending and C
O stretching vibrations of the carboxyl group (1197 and 1695 cm−1); the asymmetric stretching band of the carboxylate group (1427 cm−1); and the N–H bending and C