Influence of hydration on ion–biomolecule interactions: M+(indole)(H2O)n (M = Na, K; n = 3–6)†
Abstract
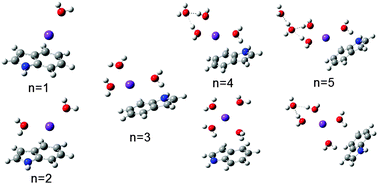
The indole functional group can be found in many biologically relevant molecules, such as neurotransmitters, pineal hormones and medicines. Indole has been used as a tractable model to study the hydration structures of biomolecules as well as the interplay of non-covalent interactions within ion–biomolecule–water complexes, which largely determine their structure and dynamics. With three potential binding sites: above the six- or five-member ring, and the N–H group, the competition between π and hydrogen bond interactions involves multiple locations. Electrostatic interactions from monovalent cations are in direct competition with hydrogen bonding interactions, as structural configurations involving both direct cation–indole interactions and cation–water–indole bridging interactions were observed. The different charge densities of Na+ and K+ give rise to different structural conformers at the same level of hydration. Infrared spectra with parallel hybrid functional-based calculations and Gibbs free energy calculations revealed rich structural insights into the Na+/K+(indole)(H2O)3–6 cluster ion complexes. Isotopic (H/D) analyses were applied to decouple the spectral features originating from the OH and NH stretches. Results showed no evidence of direct interaction between water and the NH group of indole (via a σ-hydrogen bond) at current levels of hydration with the incorporation of cations. Hydrogen bonding to a π-system, however, was ubiquitous at hydration levels between two and five.

 Please wait while we load your content...
Please wait while we load your content...