Structurally diverse diterpenoids from Isodon pharicus†
Abstract
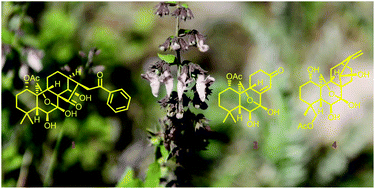
Twenty-one diterpenoids with diverse structures, including twelve new compounds (1–12), were isolated from the aerial parts of Isodon pharicus. Structurally, pharicusin A (1), composed of a benzoyl group coupled to a 7α,20-epoxy-ent-kauranoid, represents an unprecedented C27 meroditerpenoid; pharicusin B (2) is a novel trinorditerpenoid with an unreported 15,16,17-trinor-7α,20-epoxy-ent-kaurane skeleton; pharicusin C (4) represents the first example of 7α,20-epoxy-ent-atisane diterpenoids. Their structures were elucidated based on spectroscopic analysis, wherein the absolute configurations of 2 and 3 were further confirmed by single-crystal X-ray diffraction and computational analysis of the optical rotatory dispersion (ORD). Selected sixteen isolates were evaluated for their in vitro cytotoxicity against a small panel of human tumor cell lines (HL-60, SMMC-7721, A-549, MCF-7, and SW-480), and compounds 9, 10, 13, 16, and 18 exhibited significant inhibitory effects against all five cell lines, with IC50 values in the range of 0.37–6.03 μM.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...