Green carbon disulfide surrogate via a combination of potassium sulfide and chloroform for benzothiazine-thione and benzothiazole-thione construction†‡
Abstract
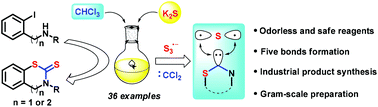
An efficient and green carbon disulfide surrogate via facile combination of potassium sulfide and chloroform has been developed. A variety of benzothiazine-thiones and benzothiazole-thiones were straightforwardly established along with the formation of five new chemical bonds in one pot from readily available starting materials, in which the widely used 2-mercaptobenzothiazole (MBT) was synthesized through this method in gram scale. Dichlorocarbene was demonstrated to be a carbon source intermediate from chloroform on the basis of control experimental studies. Meanwhile, potassium sulfide, as a trisulfur radical anion (S3˙−) source, was confirmed through UV-visible spectroscopy and an EPR study.
- This article is part of the themed collections: Celebrating the 90th birthday of Professor Lu Xiyan and Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...