Oxidation of CO on a carbon-based material composed of nickel hydroxide and hydroxyl graphene oxide, (Ni4(OH)3–hGO) – a first-principles calculation†
Abstract
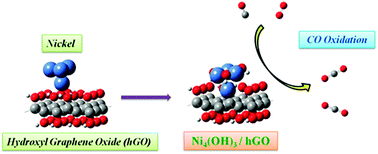
Nickel or nickel hydroxide clusters and graphene oxide (GO) composites are novel nanomaterials in the application of electrochemical catalysts. In this work, we calculated the energy of Ni4 adsorbed onto saturated hydroxyl graphene oxide (hGO), which forms a Ni4(OH)3 cluster on the hydroxyl graphene oxide (Ni4(OH)3–hGO) and releases 4.47 eV (5.22 eV with DFT-D3 correction). We subsequently studied the oxidation of CO on the Ni4(OH)3–hGO system via three mechanisms – LH, ER and carbonated mechanisms. Our results show that the activation energy for oxidation of the first CO molecule according to the ER mechanism is 0.14 eV (0.12 eV with DFT-D3 correction), much smaller than that with LH (Ea = 0.65 eV, 0.61 eV with DFT-D3 correction) and with carbonated (Ea = 1.28 eV, 1.20 eV with DFT-D3 correction) mechanisms. The barrier to oxidation of the second CO molecule to CO2 with the ER mechanism increases to 0.43 eV (0.37 eV with DFT-D3 correction), but still less than that via LH (Ea = 1.09 eV, 1.07 eV with DFT-D3 correction), indicating that CO could be effectively oxidized through the ER mechanism on the Ni4(OH)3/hGO catalyst.

 Please wait while we load your content...
Please wait while we load your content...